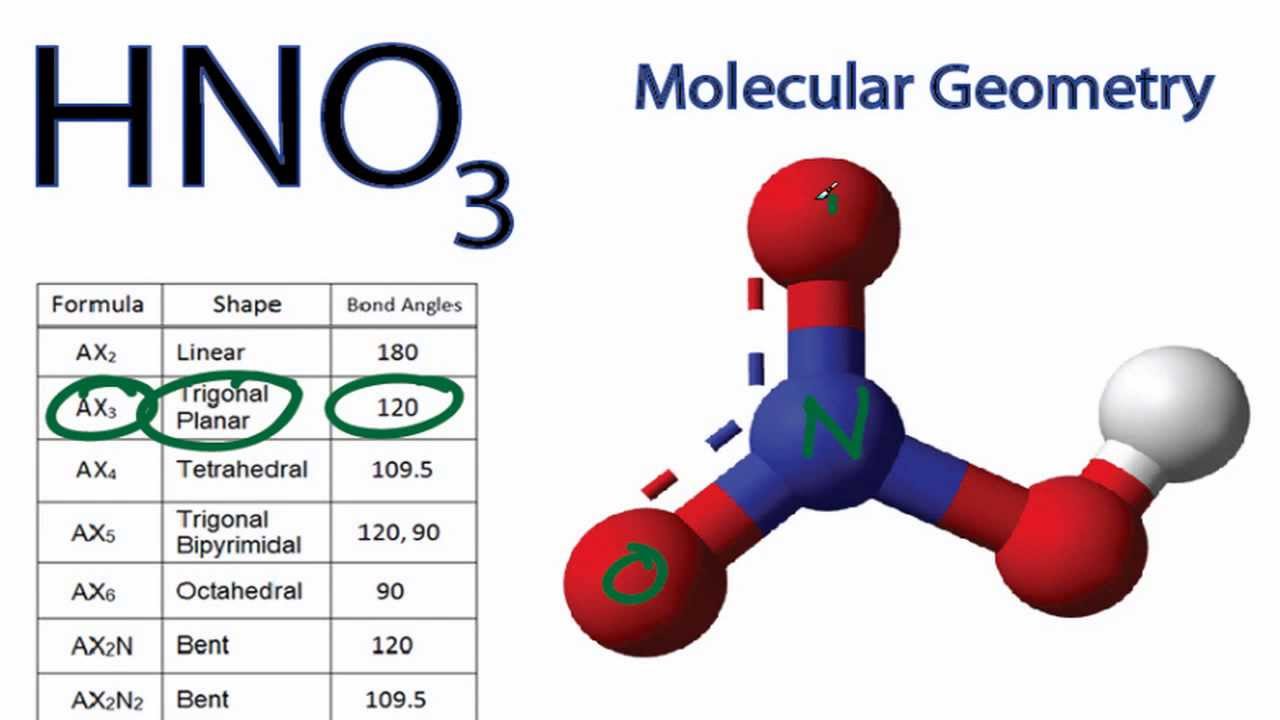
HNO3 Molecular Geometry / Shape and Bond Angles YouTube
A step-by-step explanation of how to draw the HNO3 Lewis Structure (Nitric Acid). The HNO3 Lewis structure is best thought of as the NO3 with an H attache.
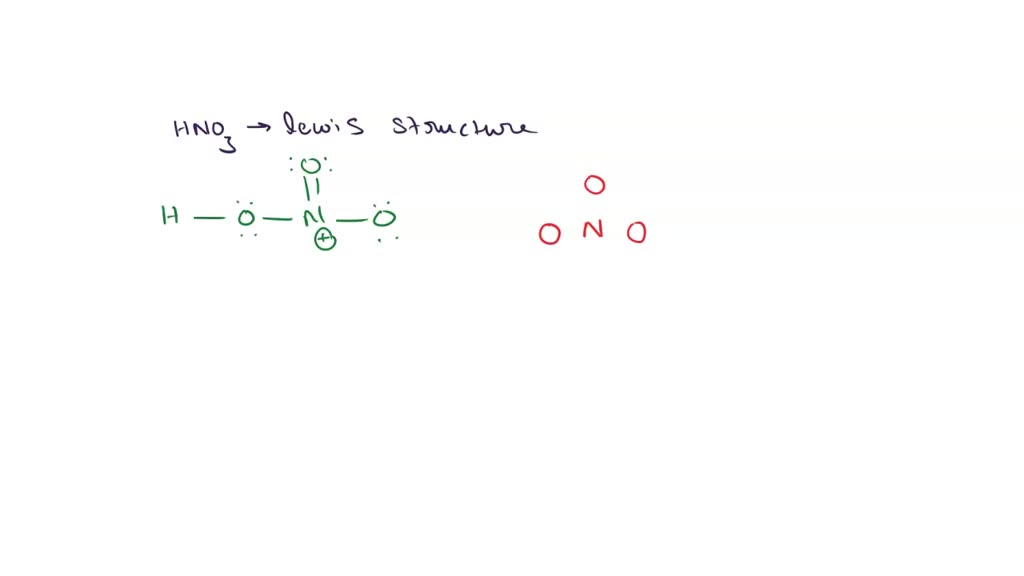
SOLVED Draw the Lewis structure for HONO2 (HNO3) (including double bonds, lone pairs, and any
Step #4: Complete the octet (or duplet) on outside atoms. If the valence electrons are left, then put the valence electrons pair on the central atom. Don't worry, I'll explain! In the Lewis structure of HNO3, the outer atoms are hydrogen atom as well as oxygen atom. Hydrogen already has a duplet (see below image).

Lewis Dot Structure of HNO3 How to Draw Lewis Structures Class 11 Chemistry Chemical
Let's discuss the HNO3 lewis structure. In the HNO3 lewis structure, the molecule is consist of N, H, and three O atoms. All the atoms in the HNO3 lewis structure make a covalent bond. The N is the HNO3 lewis structure is sp2 hybridized while O is sp3 hybridized. There is one -OH group and two ketonic groups are present.

Estructura de Lewis HNO3 (ácido nítrico) ion nitrato y nitrato de sodio YouTube
Struktur Lewis HNO3 (asam nitrat) memiliki atom nitrogen (N) di tengahnya yang dikelilingi oleh dua atom oksigen (O) dan gugus OH. Terdapat 1 ikatan rangkap antara atom nitrogen (N) dan atom oksigen (O) dan atom lainnya mempunyai ikatan tunggal. Jika Anda tidak memahami apa pun dari gambar struktur Lewis HNO3 (asam nitrat) di atas, ikuti terus.
Simple Procedure for writing Lewis Structures Lewis Structures for nitric acid (HNO3
Step 1: Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. Step 2: Connect the atoms to each other with single bonds to form a "skeleton structure.".

QUÍMICA Estructura de Lewis HNO3 Enlace Dativo Carga Formal BACHILLERATO AULAEXPRESS YouTube
A step-by-step explanation of how to draw the HNO3 Lewis Dot Structure (Nitric acid).For the HNO3 structure use the periodic table to find the total number o.

Structure of the HNO 3 molecule. Download Scientific Diagram
Steps of drawing HNO3 lewis structure Step 1: Find the total valence electrons in HNO3 molecule. In order to find the total valence electrons in a HNO3 molecule, first of all you should know the valence electrons present in hydrogen atom, nitrogen atom as well as oxygen atom. (Valence electrons are the electrons that are present in the outermost orbit of any atom.)

12+ Lewis Structure Of Hno3 Robhosking Diagram
Lawyers in our Dallas office handle matters across the firm's core practices, including labor and employment, mergers and acquisitions, white collar litigation and investigations, and securities enforcement and litigation. Our Dallas corporate and business transactions team is well-versed in advising clients on mergers and acquisitions of employee stock ownership plan (ESOP) companies and.

HNO3 Lewis Structure How to Draw the Lewis Structure for HNO3 YouTube
Steps. Use these steps to correctly draw the HNO 3 Lewis structure: #1 First draw a rough sketch. #2 Mark lone pairs on the atoms. #3 Calculate and mark formal charges on the atoms, if required. #4 Convert lone pairs of the atoms, and minimize formal charges.

Nitric Acid (HNO3) 3D Model with Lewis Structure YouTube
Nitric Acid is a strong acid with a PH of about 3.01. It is a 'sticky' molecule that readily absorbs to surfaces, specifically if there is water on the surface. The physical state of a pure nitric acid is a colorless liquid, but older samples often acquire a yellowish tint due to decomposition into nitrogen oxides and water.

Draw the Lewis structure of nitric acid, `HNO_3`. Sarthaks eConnect Largest Online Education
Step-by-Step Guide to Drawing the Lewis Structure of HNO3. 1. Determine the total number of valence electrons. Hydrogen (H) contributes 1 valence electron. Nitrogen (N) contributes 5 valence electrons. Oxygen (O) contributes 6 valence electrons each. Therefore, the total number of valence electrons in HNO3 is: (1 × 1) + 5 + (3 × 6) = 24.

Perhatikan struktur lewis molekul HNO3 berikut. H 1 O 2 N...
The HNO3 Lewis structure is best thought of as the NO3 with an H attached to one of the oxygen atoms. This is a pattern seen with many acids. For the HNO3 Lewis structure, calculate the total number of valence electrons for the HNO3 molecule. After determining how many valence electrons there are in HNO3, place them around the central atom to.

Lewis Dot Structure For Hno3 Draw Easy
This chemistry video tutorial explains how to draw the lewis structure of HNO3 - Nitric Acid.How To Draw Lewis Structures: https://www.youtube.co.

El Diagrama De Lewis
Drawing the Lewis Structure for HNO 3. The HNO 3 Lewis structure is easier to think of if you consider it NO 3 with an H bonded to one of the oxygen atoms. In HNO 3 Lewis structure Nitrogen (N) is the least electronegative atom and goes in the center of the Lewis structure. Check the formal charges to be sure that each atom has a formal charge.

Estructura de lewis HNO3, enlace covalente normal polar y dativo YouTube
There are two resonance structures HNO3 (Nitric Acid). We start with a valid Lewis structure and then follow these general rules. For the HNO3 resonance stru.

La estructura de Lewis del HNO3 Complex Solutions
HNO3 Lewis Structure, Molecular Geometry, Hybridization, and MO Diagram. Nitric acid (HNO3), a highly corrosive acid, is a very important chemical. It is usually a colorless liquid, but the older samples turn pale yellow because it gets decomposed into water and oxides of nitrogen. This toxic liquid has yellow or red-brown fumes that can cause.