Kimia Kelas 11 Pengertian Isomer, Jenisjenisnya, Serta Contohnya Belajar Gratis di Rumah
Main page; Contents; Current events; Random article; About Wikipedia; Contact us; Donate

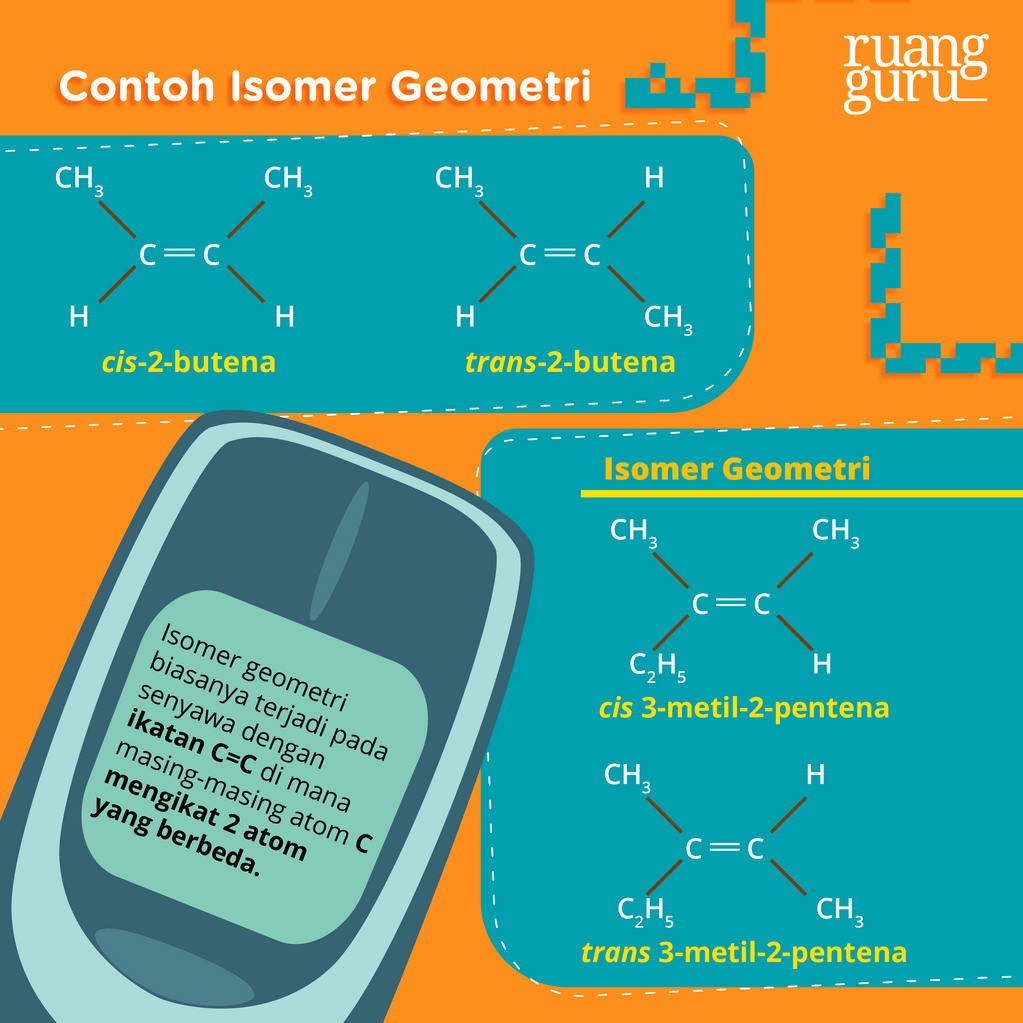
Isomer Alkana, Alkena, Alkuna Pengertian, Jenis, Jumlah dan Namanya
Generate the Isomers of Decane. There are 75 isomers of decane, with the chemical formula . This example shows how to start with methane and add carbon atoms one by one to exhaustively list all alkanes with up to 10 carbon atoms. Write a function to take a molecule and replace each unique group with a group, returning the results as a list.

ISOMER DEKANA YouTube
Now we add enough hydrogen atoms to give each carbon four bonds. Figure 8.3.1 8.3. 1 ). Figure 8.3.1 8.3. 1: Butane and Isobutane. The ball-and-stick models of these two compounds show them to be isomers; both have the molecular formula C 4 H 10. Notice that C 4 H 10 is depicted with a bent chain in Figure 8.3.1 8.3. 1.
Isomer wikidoc
Decane is a hydrocarbon with the molecular formula C10H22. It belongs to the alkane family and is composed of ten carbon atoms bonded together with 22 hydrogen atoms.One interesting aspect of decane is its isomers, which are compounds with the same molecular formula but different structural arrangements.Isomers of decane can vary in terms of the position of the carbon-carbon bonds, resulting.
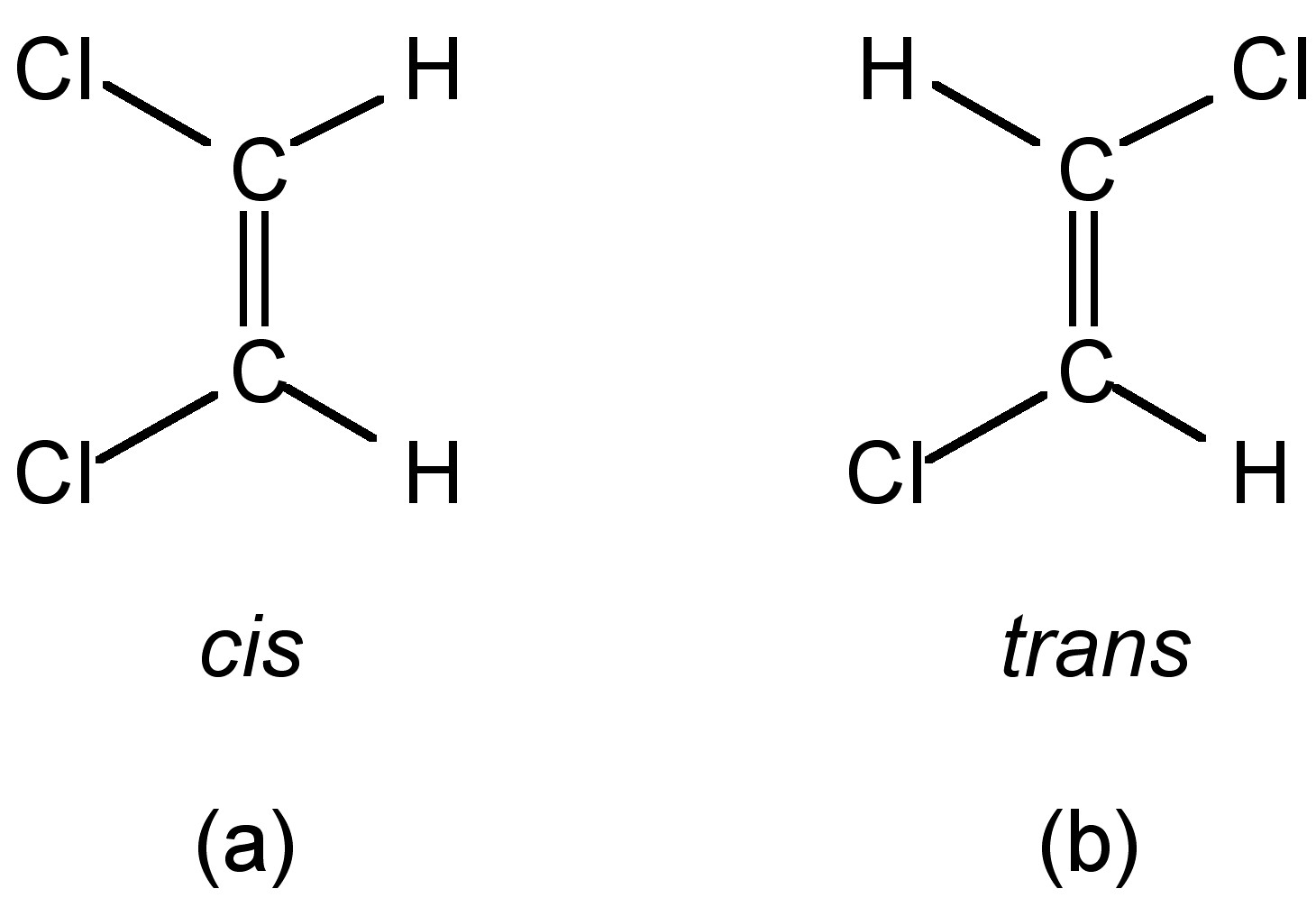
World of Biochemistry (blog about biochemistry) Geometric isomers
Isomer Dekana | PDF. Scribd is the world's largest social reading and publishing site.

Organic Molecules and Isomers Biology 201 The Chemistry of Life
Table source: "12.5: IUPAC Nomenclature" In Basics of GOB Chemistry (Ball et al.), CC BY-NC-SA 4.0. An alkyl group is a group of atoms that results when one hydrogen atom is removed from an alkane. The group is named by replacing the -ane suffix of the parent hydrocarbon with -yl.For example, the -CH 3 group derived from methane (CH 4) results from subtracting one hydrogen atom and is.
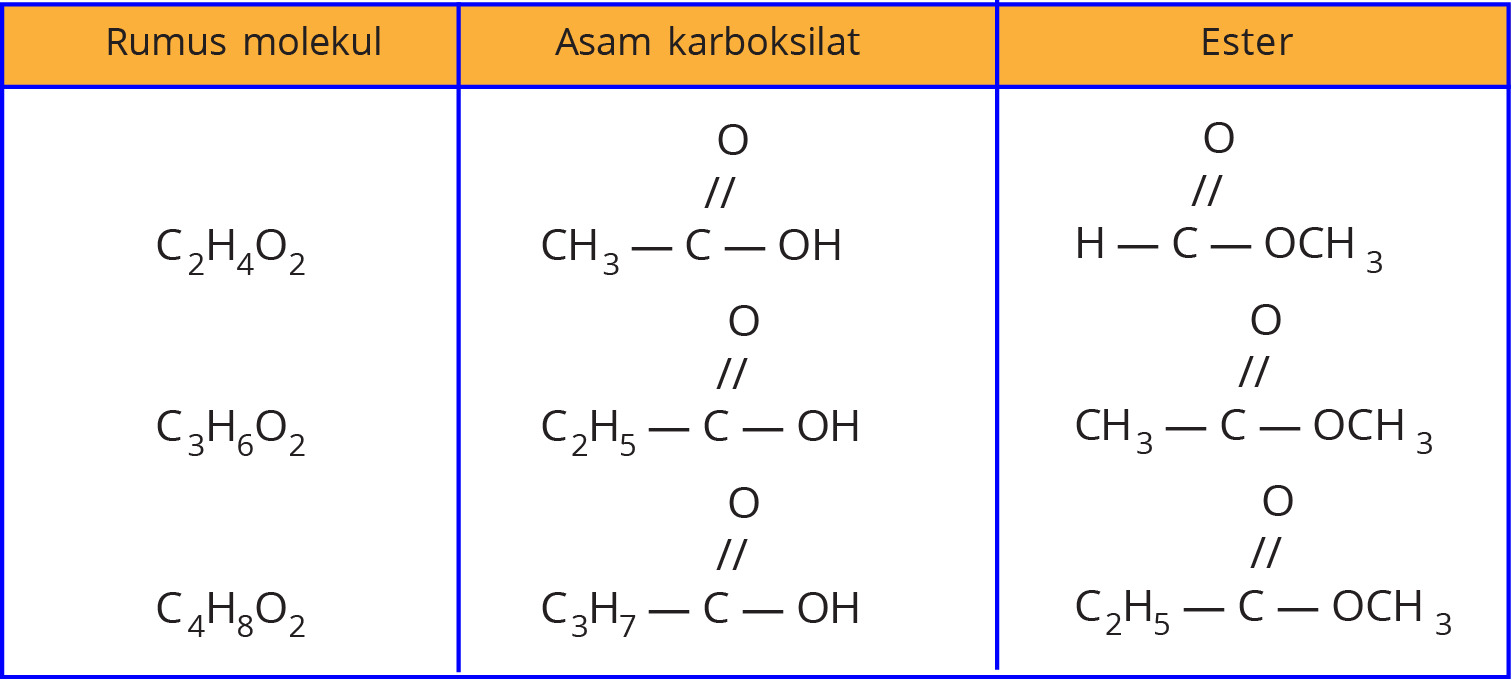
Isomer Asam Alkanoat dan Alkil Alkanoat Materi Kimia
3.2 Alkanes and Alkane Isomers. Before beginning a systematic study of the different functional groups, let's look first at the simplest family of molecules to develop some general ideas that apply to all families. We saw in Section 1.7 that the carbon-carbon single bond in ethane results from σ (head-on) overlap of carbon sp3 hybrid orbitals.
Isomer Organic Chemistry Video Clutch Prep
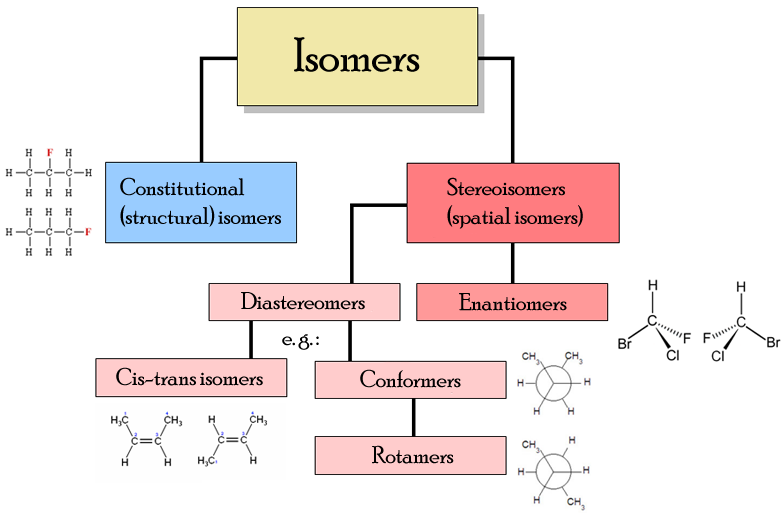
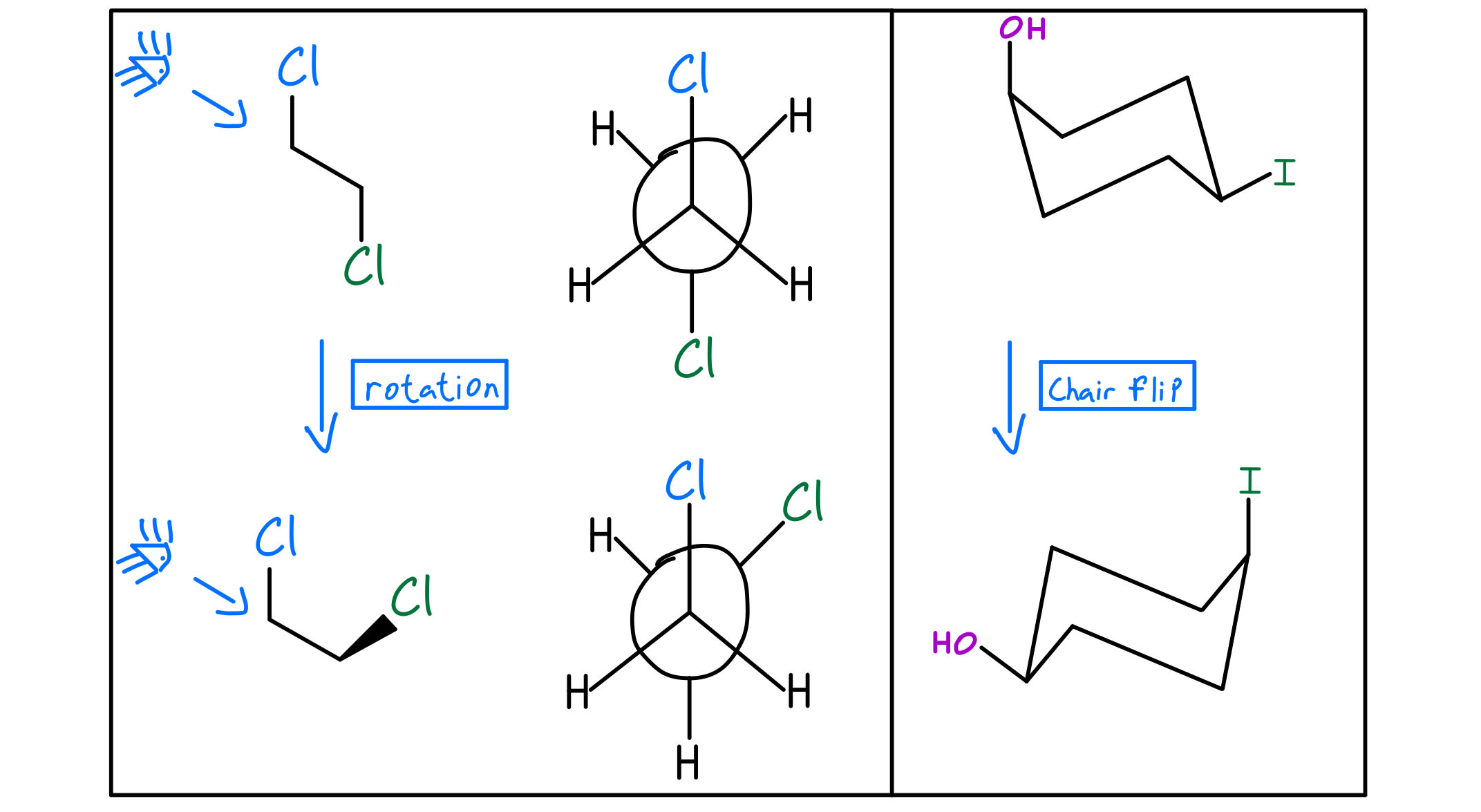
Isomers. Isomers are compounds with the same molecular formulae but different structural formulae. They have the same number of each type of atom but may have different physical and chemical.

Isomer Sikloalkana (Kimia SBMPTN, UN, SMA) YouTube
An isomer is the ability for a molecule to change its structure while keeping its chemical formula the same. Think of isomers as a structural rearrangement of a particular compound. An example of.
2 Isomers Honors Chemistry Online
Decane, a 10-carbon n-alkane and one of the highest vapor phase constituents of jet propellent-8 (), was selected to represent the semivolatile fraction for the initial development of a physiologically based pharmacokinetic (PBPK) model for JP-8.Rats were exposed to decane vapors at time-weighted average concentrations of 1200, 781, or 273 ppm in a 32-L Leach chamber for 4 hr. Time-course.

Ch 7 Isomer types
List of isomers of decane . Hydrocarbons; Isomerism; Lists of isomers of alkanes; This is the list of the isomers of decane. Contents. 1 Straight-chain; 2 Nonane; 3 Octane. 3.1 Ethyl; 3.2 Dimethyl; 4 Heptane. 4.1 Propyl; 4.2 Methyl+Ethyl; 4.3 Trimethyl; 5 Hexane. 5.1 Methyl+Propyl; 5.2 Diethyl; 5.3 Dimethyl+Ethyl;

Position and functional group isomers
2.2: Alkanes and Alkane Isomers is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by LibreTexts. Alkanes are organic compounds that consist entirely of single-bonded carbon and hydrogen atoms and lack any other functional groups. Alkanes have the general formula CnH2n+2 and can be subdivided..
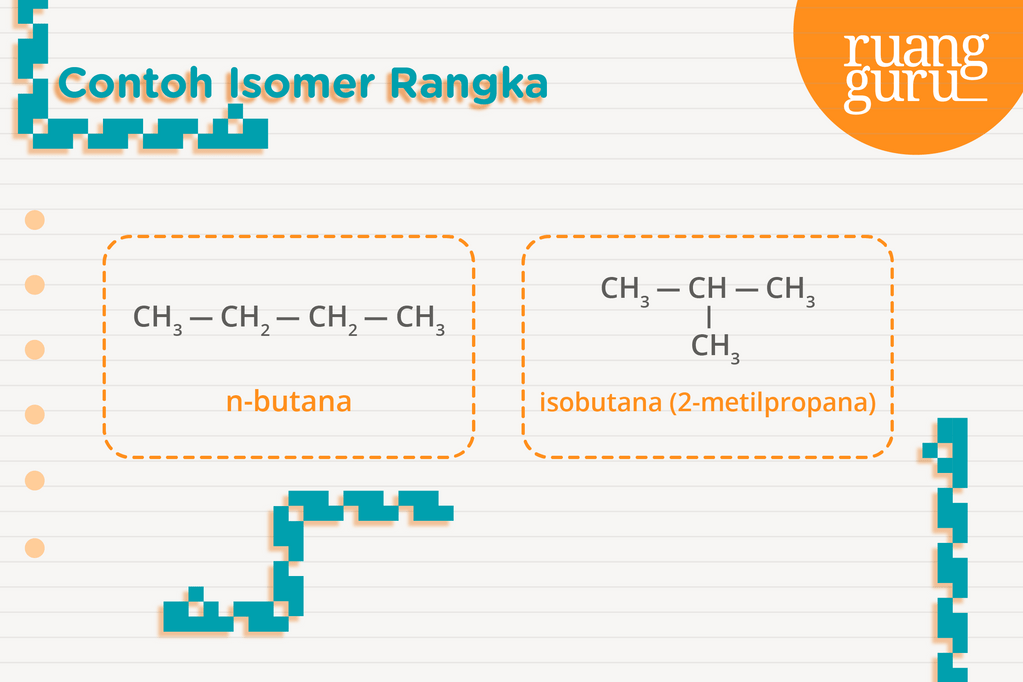
Kimia Kelas 11 Pengertian Isomer, Jenisjenisnya, Serta Contohnya Belajar Gratis di Rumah
Decane is an alkane hydrocarbon with the chemical formula C 10 H 22.Although 75 structural isomers are possible for decane, the term usually refers to the normal-decane ("n-decane"), with the formula CH 3 (CH 2) 8 CH 3.All isomers, however, exhibit similar properties and little attention is paid to the composition. These isomers are flammable liquids.Decane is present in small quantities (less.

A Brief Guide to Types of Isomerism in Organic Chemistry Compound Interest
Conformational Isomers. The C-C single bonds in ethane, propane, and other alkanes are formed by the overlap of an sp 3 hybrid orbital on one carbon atom with an sp 3 hybrid orbital on another carbon atom, forming a σ bond. Each sp 3 hybrid orbital is cylindrically symmetrical (all cross-sections are circles), resulting in a carbon-carbon single bond that is also cylindrically symmetrical.
Jumlah Isomer Alkuna Dengan Rumus Molekul C5H8 Adalah Lengkap
Original source: https://en.wikipedia.org/wiki/List of isomers of decane. Read more

Contoh Soal Isomer Homecare24
Consider the molecular formula. C7H7Cl (3.4.3) (3.4.3) C 7 H 7 C l. There are four different isomers you could make depending on the position of the chlorine atom. In one case it is attached to the side-group carbon atom, and then there are three other possible positions it could have around the ring - next to the.