
Write structure of the isomer of pentane that on chlorination gives only one monochloro
Conformational isomers, also known as conformers, differ from one another by their rotation around a single bond. Rotations occur freely around single carbon-carbon bonds. Unlike double and triple bonds, which are "locked" in their orientation, single bonds have no such restrictions. Figure 5.1.2 5.1. 2: Conformational isomers of pentane.
Kimia Kelas 11 Pengertian Isomer, Jenisjenisnya, Serta Contohnya Belajar Gratis di Rumah
Isomers of pentanol are a group of organic compounds that have the same molecular formula, C5H12O, but differ in their structural arrangement. Pentanol is an alcohol with five carbon atoms and a hydroxyl group (-OH). The isomers of pentanol can be classified into three main types: n-pentanol, iso-pentanol, and neo-pentanol.

please giv the possible isomers of pentane and their names also Science Carbon and its
1. Draw the structures of isomers of : pentane. View Solution. Q 2. How many structures of isomers can you draw for pentane? View Solution. 3. Shiva asked surabhi to draw the structural isomers of pentane.

Pentane, isopentane and neopentane are a type of
Isomers of pentane are a fascinating topic in organic chemistry. Pentane is a hydrocarbon with the molecular formula C5H12, and it consists of five carbon atoms bonded together with twelve hydrogen atoms.However, what makes pentane interesting is that it can exist in different structural forms known as isomers. Isomers have the same molecular formula but differ in the arrangement of atoms.

Draw the structural formula for the following An isomer of npentane 10 SELF ASSESSMENT PAP
Consider the molecular formula. C7H7Cl (3.4.3) (3.4.3) C 7 H 7 C l. There are four different isomers you could make depending on the position of the chlorine atom. In one case it is attached to the side-group carbon atom, and then there are three other possible positions it could have around the ring - next to the.

Buatlah Isomer Dari Pentana Lengkap Dengan Rumus Struktur Dan Namanya My XXX Hot Girl
Isomer pentuna merupakan senyawa organik dengan rumus C5H10 dan memiliki lima Pembaca Sekalian, pada kesempatan kali ini kita akan membahas isomer pentuna. Beranda
[Solved] 2. Draw all of the structural isomers for a) pentane b) hexane c)... Course Hero
Exercise 3.2.7 3.2. 7. Draw the 5 constitutional isomers of C 7 H 16 (of the 9 total isomers possible) that have 5 carbons as the longest carbon chain length. Answer. Alkanes are organic compounds that consist entirely of single-bonded carbon and hydrogen atoms and lack any other functional groups. Alkanes have the general formula CnH2n+2 and.
Show the isomers of Pentene nbsp;
Pentane is a nonpolar liquid that cannot be dissolved in water because of its hydrophobic nature. It is known to be less dense than water; its density is equal to 0.6262 g/mL at 20 ∘ C. For.

[Carbon and it's componds] What are isomers? Class 10 Teachoo
The isomers of pentyne refer to the different structural arrangements of the compound pentyne, which is a hydrocarbon with five carbon atoms and multiple bonds. Isomers are molecules that have the same molecular formula but differ in the arrangement of atoms. In the case of pentyne, there are three possible isomers: 1-pentyne, 2-pentyne, and 3.
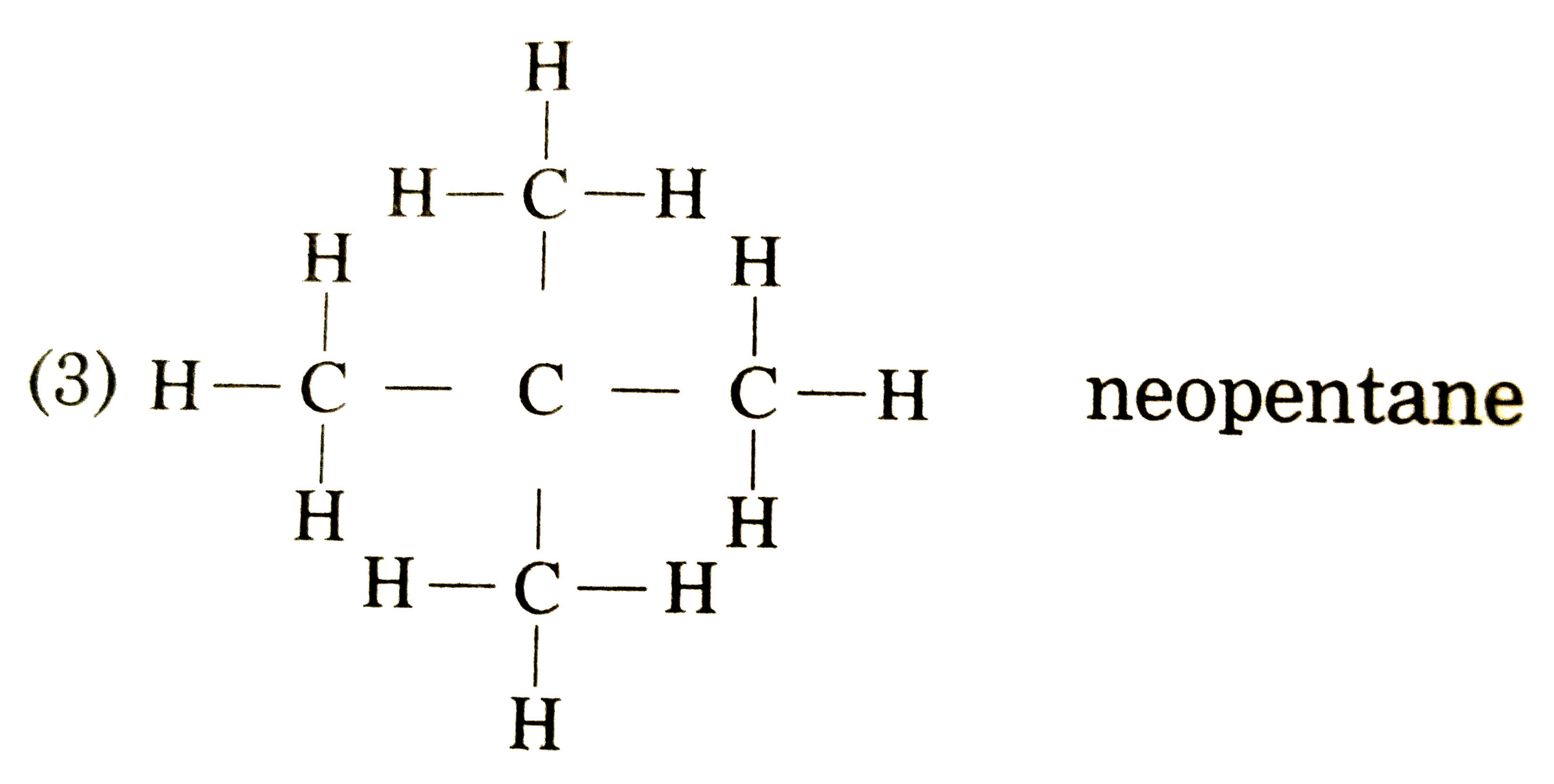
Draw the structures of isomers of pentane (C(5)H(12)).
Pentane is an organic compound with the formula C 5 H 12 —that is, an alkane with five carbon atoms. The term may refer to any of three structural isomers, or to a mixture of them: in the IUPAC nomenclature, however, pentane means exclusively the n-pentane isomer, in which case pentanes refers to a mixture of them; the other two are called isopentane (methylbutane) and neopentane.

pentene isomers Chemistry notes, Organic chemistry notes, Chemistry
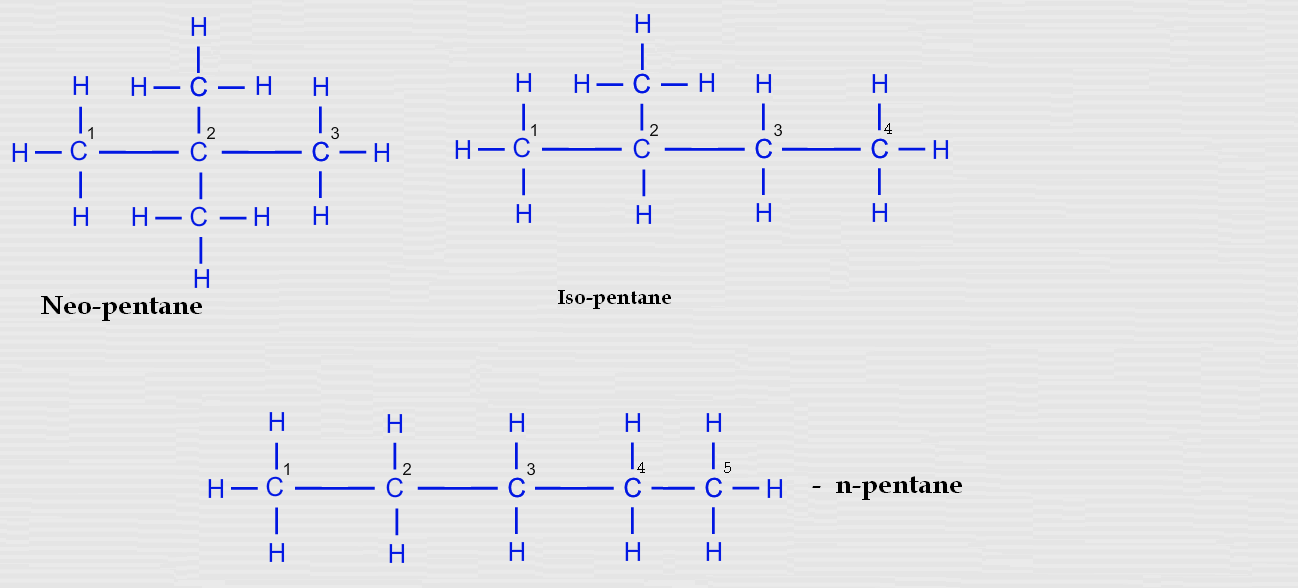
Products from Oil. What are the Isomers of Pentane?. Pentane (C 5 H 12) has three structural isomers.. Isomer 1 is n-pentane, the straight chain normal structure for pentane.. Isomer 2 is 2-methylbutane, a branched chain with a carbon* atom joined onto three other carbon atoms.. Isomer 3 is 2,2-dimethylpropane, a branched chain with the central carbon* atom joined onto four other carbon atoms.

How many structural isomers can you draw for pentane? Sarthaks eConnect Largest Online
Memberikan nama sesuai urutan penamaan yaitu "nomor cabang-nama cabang-nomor ikatan rangkap 3-nama rantai utama". Senyawa pentuna pada soal, mempunyai isomer posisi yaitu 1-pentuna dan 2-pentuna. 1-pentuna. 2-pentuna. Senyawa pada soal, juga mempunyai isomer rantai yaitu 1-pentuna dan 3-metil-1-butuna. 1-pentuna. 3-metil-1-butuna.

How many structural isomers can you draw for pentane?
Vacuum ultraviolet photoabsorption spectra of pentane (C 5 H 12) structural isomers (n-pentane and isopentane) are recorded in the 6-11.5 eV region using a synchrotron light source.The photoabsorption spectra for both the pentane isomers showed a continuum, beginning at about 7.5 eV and increasing in intensity up to 10.5 eV with few broad peaks devoid of discernible structures.

Organic Molecules and Isomers Biology 201 The Chemistry of Life
Isomers of pentene refer to a group of organic compounds that have the same molecular formula, C5H10, but differ in their structural arrangement.Pentene is a hydrocarbon with five carbon atoms and ten hydrogen atoms, and it exists in various isomeric forms.These isomers can be classified into three types: 1-pentene, 2-pentene, and 3-pentene.Each isomer has a unique arrangement of carbon atoms.

How many structure isomers can you draw for pentane? (1) 2 (2) 4 (3) 3 (4) 6 Chemistry Q&A
Pentane is an organic compound with five carbons, and since it ends in -ane, we can conclude that there are only single bonds in it (it is a saturated molecule). It has a molecular formula C 5 H 12. Pentane exists in three structural isomers: n-pentane, isopentane, and neopentane. In n-pentane or pentane the five carbon atoms are organized in a.
1 Pentene Structural Formula
Two molecules which have the same molecular formula but different structural formulas, or bonding arrangements, are known as constitutional isomers. Pentane is an alkane with five carbon atoms. It has three constitutional isomers, shown below. Figure 1.2.2 1.2.