
LDT Validation
The proposed rule states that "FDA has generally considered an LDT to be an IVD that is intended for clinical use and that is designed, manufactured and used within a single laboratory that is certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA) and meets the regulatory requirements under CLIA to perform high.

[Infographic] A Visual History of LDT Regulation Amplion
Keep yourself up-to-date with the newest product releases, technical content, and upcoming events. Zone in with Zon Blog, your ultimate source for the latest trends in nucleic acid research, along with our Research Update monthly.

LDT based processes
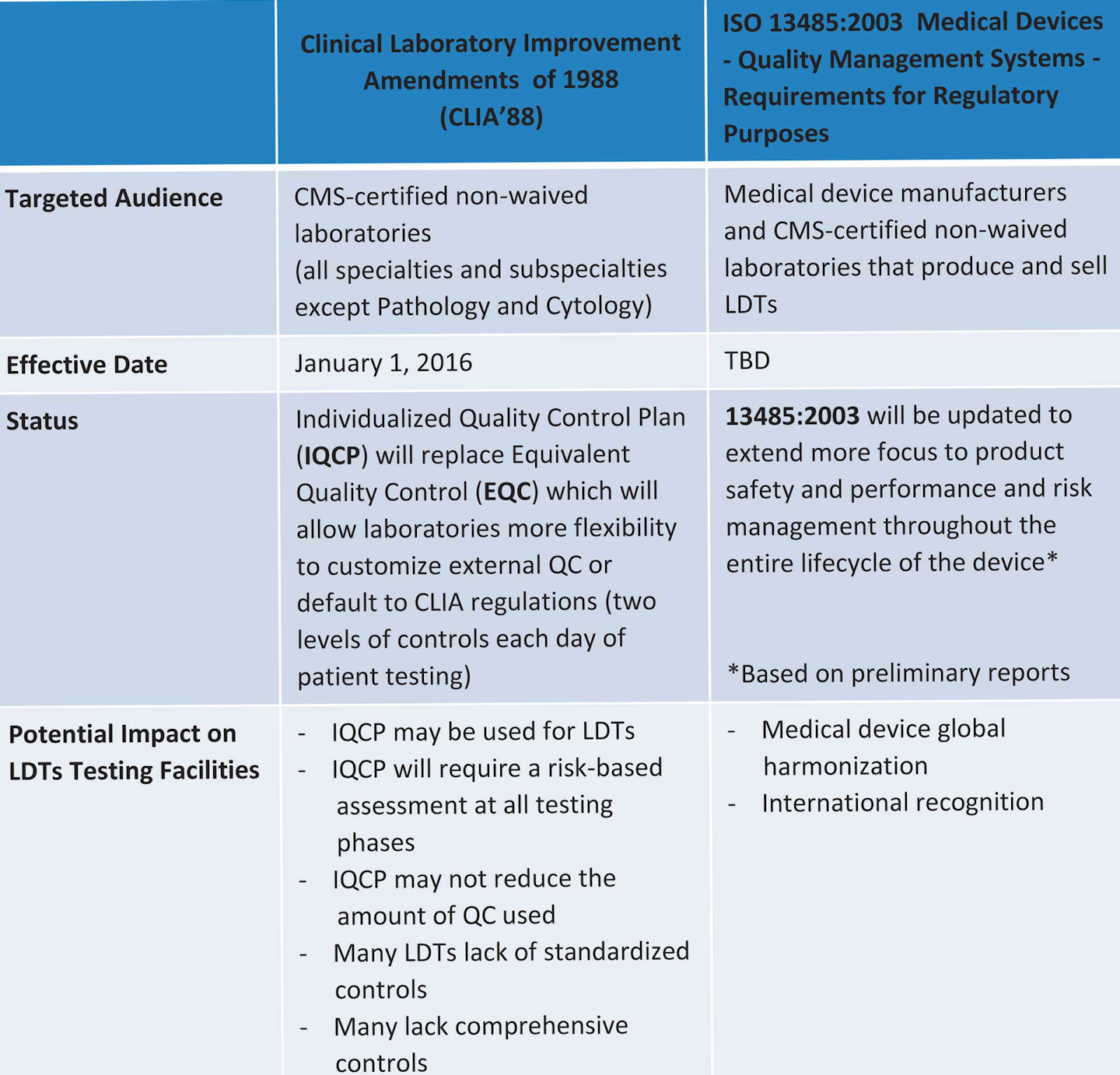
Like other medical devices, IVDs are subject to premarket and postmarket controls. IVDs are generally also subject to categorization under the Clinical Laboratory Improvement Amendments (CLIA '88) of 1988. The U.S. regulations define four other IVD types: RUO, IUO, GPR and, ASR products.

LDT
Lexical Decision Task (LDT) adalah tes yang digunakan untuk mengukur kecepatan dan akurasi deteksi bahasa. Proses dan desain LDT penting untuk memastikan bahwa data yang dihasilkan valid dan dapat diandalkan. LDT sering digabungkan dengan teknik lain untuk mempelajari bagaimana otak memproses informasi bahasa.

LDT File What is an .ldt file and how do I open it?
An LDT is a diagnostic test for clinical use that is designed, manufactured, and performed by an individual laboratory. If a clinical laboratory develops its own assay and uses it for health screening or diagnostic purposes, the FDA considers the test an LDT as long as it is not transferred, licensed, or sold to other laboratories.

Lexical Decision Task (LDT) Psikologi Kognitif LEXICAL DECISION TASK Lexical Decision Task
LDT additions under an exemption are still subject to risk-based review. A currently enacted model therefore exists outside the FDA for LTD submission and review, with a carve-out for exemptions when a laboratory has previously demonstrated proficiency with assay validation and operation using that methodology. A more extensive evaluation of.
The push to reconcile the gap in LDT regulations Medical Laboratory Observer
Clinical laboratory results influence more than 70 % of medical decisions and are a critical component of healthcare. Clinical laboratory tests may be manufactured commercially or developed within a clinical laboratory setting. In the current landscape, commercially manufactured assays, which are considered in vitro diagnostic (IVD) devices, go.

Jual Meja Operasi Elektrik LDT100A PT. DUMEDPOWER INDONESIA
Tests offered as LDTs—including those that fall outside of FDA's historically narrow definition of what constitutes an LDT (i.e., a test intended for clinical use that is designed, manufactured and used within a single high-complexity Clinical Laboratory Improvement Amendments (CLIA) laboratory)—may remain on the market until FDA.

[김세우의LDT리더십]제6강. 가치관과 팀 빌딩.배우고 행동하고 가르치는 LDT 리더십 저자특강 김세우강사 YouTube
FDA is under no obligation to publish the LDT rule according to the schedules reflected in the Unified Agenda. If the rule and related LDT policy are finalized as proposed by April 2024, high-risk LDTs may be called-in for premarket review as early as October 1, 2027.

LDT Flowchart Chikly Health Institute
The Tenth District Alternative Dispute Resolution Program provides an alternative method of resolving disputes in cases filed in the Superior, State, Probate and Magistrate courts of Athens-Clarke County. This program also coordinates the Divorcing Parents Program.

배우고 행동하며 가르치는 LDT 리더십 8강. 뜻하는 바를 현실로 만드는 신념과 의지 / ldt리더십연구소 YouTube
OvaSure was an LDT intended to be used to identify high-risk women who might have ovarian cancer. It was later determined that 1 in 15 women with a positive test would in fact have ovarian cancer while the other 14 had false-positive tests. There have been several other similar instances involving LDTs through the years.

activation of LDTNAc glutamatergic inputs during... Download Scientific Diagram
LDT system adalah singkatan dari Laras dalam tabung, dimana laras tersimpan dibalam tabung angin, membuat irit angin , laras juga lebih stabil tdk keganggu .
LDT Editor
PRESS RELEASE / 02.06.2024 - Transocean Ltd. Announces Fourth Quarter, Full Year 2023 Earnings Release Date read more >. PRESS RELEASE / 12.12.2023 - Transocean Ltd. Announces $251 Million Harsh Environment Semisubmersible Contract read more >. PRESS RELEASE / 10.30.2023 - Transocean Ltd. Reports Third Quarter 2023 Results read more >.

EU IVDR Compliance for LDT and IHIVD Tests Oriel STAT A MATRIX
HyperTransport (HT), sebelumnya dikenal sebagai Lightning Data Transport (LDT), adalah sebuah saluran komunikasi dua arah (bidirectional) yang dapat mentransmisikan data secara serial atau paralel serta memiliki kecepatan tinggi dan latency yang rendah. Bus ini diperkenalkan pada tanggal 2 April 2001.Adalah sebuah konsorsium yang disebut sebagai HyperTransport Consortium yang bertugas untuk.

LDT error versus time under three different mobility models for large... Download Scientific
Light displacement (LDT) is defined as the weight of the ship excluding cargo, fuel, water, ballast, stores, passengers, crew, but with water in boilers to steaming level. Normal displacement. Normal displacement is the ship's displacement "with all outfit, and two-thirds supply of stores, ammunition, etc., on board."
LDT YouTube
Berikut istilah - istilah mengenai bobot atau berat pada kapal laut adalah sebagai berikut : 1. Gross Register Tonnage (GRT) Adalah jumlah dari semua volume ruangan kapal yang tertutup atau yang dapat ditutup secara kedap air, baik yang berada dibawah geladak maupun yang berada diatasnya. 2. Nett Register Tonnage (NRT)