
Find oxidation number of S in PbSO4
Lead(II) sulfate (PbSO 4) is a white solid, which appears white in microcrystalline form.It is also known as fast white, milk white, sulfuric acid lead salt or anglesite.. It is often seen in the plates/electrodes of car batteries, as it is formed when the battery is discharged (when the battery is recharged, then the lead sulfate is transformed back to metallic lead and sulfuric acid on the.

Observation by ESEMEDX of PbSO4, CdO, and CuO particles encountered in... Download Scientific
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others. Lead (II) sulfate 98%; CAS Number: 7446-14-2; EC Number: 231-198-9; Synonyms: Anglesite; Linear Formula: PbSO4; find Sigma-Aldrich-307734 MSDS, related peer-reviewed papers.
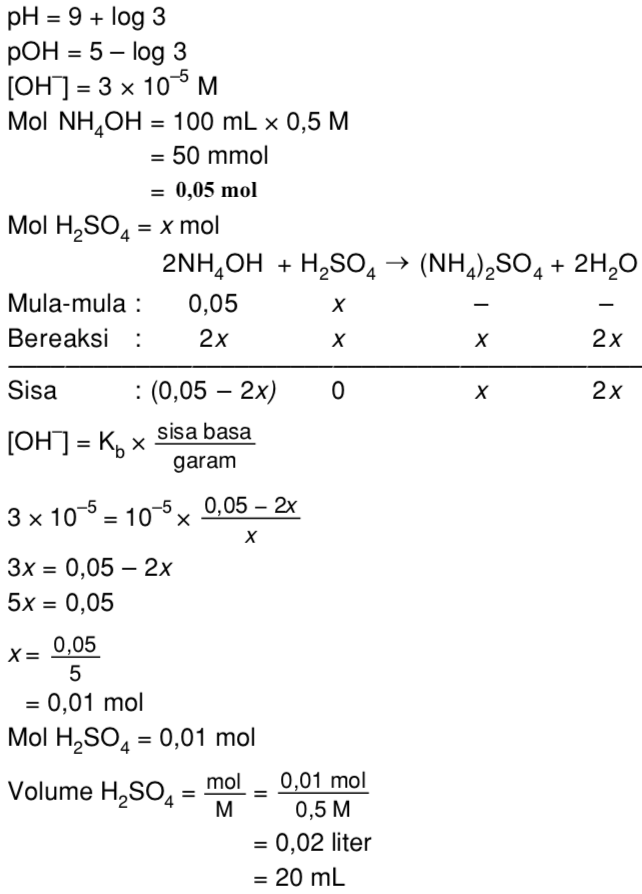
Berapa mL larutan H2SO4 0,5 M yang harus ditambahkan ke dalam 100 mL larutan NH4OH 0,5 M Mas Soal
In this video we'll write the correct formula for Lead (II) sulfate, PbSO4.To write the formula for Lead (II) sulfate we'll use the Periodic Table, a Common.

Mr.K
Anglesite is a lead sulfate mineral with the chemical formula PbSO 4.It occurs as an oxidation product of primary lead sulfide ore, galena.Anglesite occurs as prismatic orthorhombic crystals and earthy masses, and is isomorphous with barite and celestine.It contains 74% of lead by mass and therefore has a high specific gravity of 6.3. Anglesite's color is white or gray with pale yellow streaks.

PPT Chapter 4 PowerPoint Presentation, free download ID5402329
Since there is an equal number of each element in the reactants and products of PbSO4 = Pb + SO4, the equation is balanced. Balance PbSO4 = Pb + SO4 Using Inspection The law of conservation of mass states that matter cannot be created or destroyed, which means there must be the same number atoms at the end of a chemical reaction as at the.

PbSO4 LeadII Sulfate CAS 7446142 Chemical Substance in White Plastic Laboratory Packaging
Tentukan Mr dari PbSO4 - 39254257. ekai2471 ekai2471 07.03.2021 Kimia Sekolah Menengah Atas terjawab Tentukan Mr dari PbSO4 1 Lihat jawaban Iklan Iklan Hanyacatatann Hanyacatatann ~Hanyacatatann. Pendahuluan. tentukan Mr dari PbSO4 _____ Pembahasan. Diketahui : Ar Pb = 207; Ar S = 32; Ar O = 16; Ditanya :.
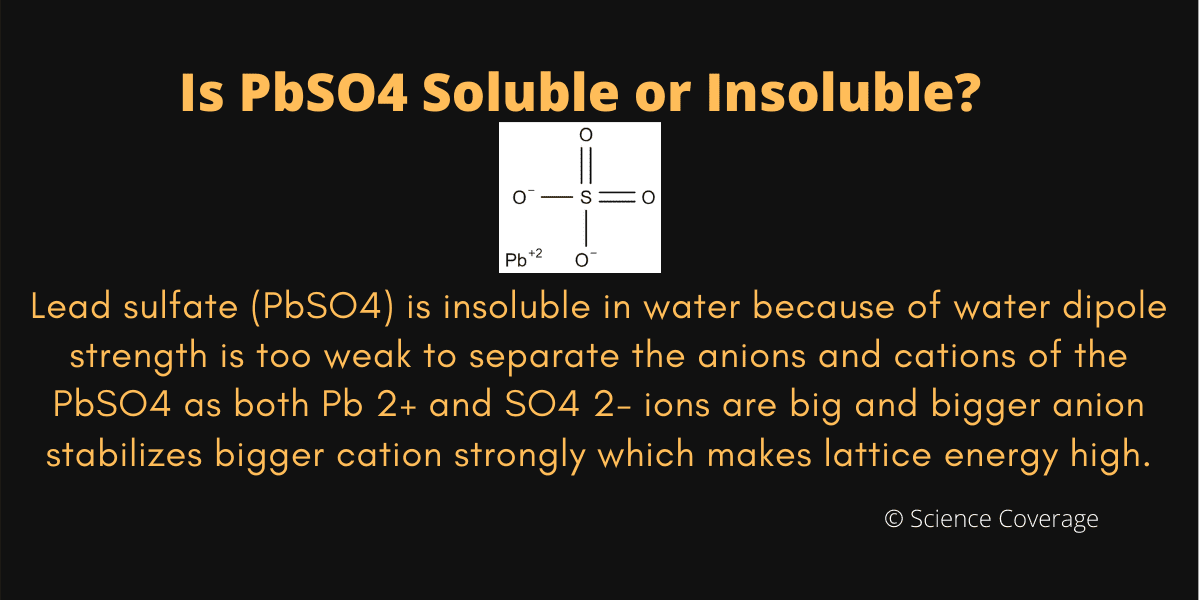
Is PbSO4 Soluble or Insoluble in Water?
M N−1. In chemistry, the molar mass ( M) of a chemical compound is defined as the ratio between the mass and the amount of substance (measured in moles) of any sample of said compound. [1] The molar mass is a bulk, not molecular, property of a substance. The molar mass is an average of many instances of the compound, which often vary in mass.

XRD patterns of (a) Ag2S/PbSO4 (b) PbSO4 nanoparticles. Download Scientific Diagram
Shows how solubility product constants for minerals can be used to compare how much of a given ion might dissolve into a solution; uses Pb (as Galena [PbS] a.

PbS+H2O2 =>PbSO4 + H2O balancing by oxidation number method. Brainly.in
Step 4: Substitute Coefficients and Verify Result. Count the number of atoms of each element on each side of the equation and verify that all elements and electrons (if there are charges/ions) are balanced. MgSO4 + Pb = Mg + PbSO4. Reactants. Products.

Equation for PbSO4 + H2O Lead (II) sulfate + Water YouTube
Compute Mass of Each Element. Multiply the number of atoms by the atomic weight of each element found in steps 1 and 2 to get the mass of each element in PbSO4: Molar Mass (g/mol) Pb (Lead) 1 × 207.2 = 207.2. S (Sulphur/Sulfur) 1 × 32.065 = 32.065. O (Oxygen)

MSDS PbSO4 PDF Toxicity Biodegradation
So, Molar mass of PbSO4 = Molar mass of 1 Lead (Pb) atom + Molar mass of 1 Sulfur (S) atom + Molar mass of 4 Oxygen (O) atoms. = 207.2 + 32.06 + (15.999) 4 = 207.2 + 32.06 + 63.996 = 303.256 g/mol. Hence the Molar mass of PbSO4 is 303.256 g/mol. I hope you have understood the short and simple calculation for finding the molar mass of PbSO4.

How to Balance PbSO4 = PbSO3 + O2 YouTube
The $\ce{PbSO4}$ formed at the anode is in solid state. Hence, writing it as $\ce{Pb^2+(s)}$ is incorrect, as it is not dissociated into the ions $\ce{Pb^2+}$ and $\ce{SO4^2-}$. With all these corrections, your final, correct cell representation should be:
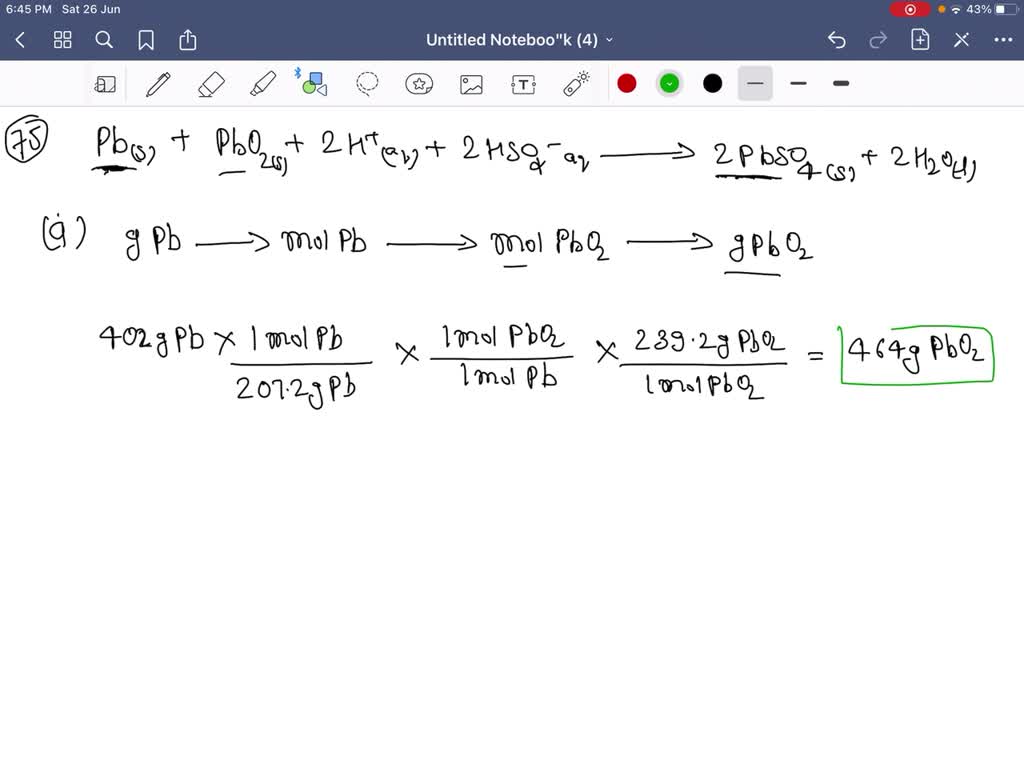
SOLVEDDuring a period of discharge of a leadacid battery, 402 g of Pb from the anode is
Explanation of how to find the molar mass of PbSO4: Lead (II) sulfate.A few things to consider when finding the molar mass for PbSO4:- make sure you have the.

PbS reacts with ozone (03) and forms PbSO4.molecules of ozone required for every one molecule of
The lead content on the skin surface of 10 lead-battery workers was measured by the method of skin stripping, and urinary lead content of rats was measured with epicutaneous application of four lead compounds: lead sulfate, lead oxide, lead powder, and lead stearate.There were significant amounts of lead on the 9th and 10th skin strippings of the dorsal hand and the back of lead workers.

Lead (II) Sulfate (PbSO4) PUDAK CTL 80, 1Gr Dunia Kimia Lestari
PbSO4 crystallizes in the orthorhombic Pnma space group. The structure is three-dimensional. Pb2+ is bonded in a 10-coordinate geometry to ten O2- atoms. There are a spread of Pb-O bond distances ranging from 2.60-3.07 Å. S6+ is bonded in a tetrahedral geometry to four O2- atoms. There are a spread of S-O bond distances ranging from 1.47-1.51 Å.

maximum number of mole of PbSO4 that can be precipitate by mixing 20ml of 0 1M Pb(NO3)2 and 30ml
Molar Mass, Molecular Weight and Elemental Composition Calculator. Molar mass of PbSO4 (Lead (II) sulfate) is 303.2626 g/mol. Get control of 2022! Track your food intake, exercise, sleep and meditation for free. Convert between PbSO4 weight and moles. Compound. Moles.