Estrutura De Lewis So3 MATERILEA
DIFFERENTIAL EQUATIONS LW.Knowles and R.T. Lewis (Editors) 0 Elsevier Science Publishers B.V. (North-Holland), 1984 7 ON POSITIVE SOLUTIONS OF ELLIPTIC EQUATIONS WITH PERIODIC COEFFICIENTS IN En,SPECTRAL RESULTS AND EXTENSIONS TO ELLIPTIC OPERATORS ON RIEMANNIAN MANIFOLDS Institute of Mathematics Hebrew University of Jerusalem Jerusalem, Israel Shmuel Agmon 1.

Lewis Dot Structure for SO3 (Sulfur trioxide) YouTube
Molecular geometry is the three-dimensional structure of the atoms which helps in the constitution of a molecule. It can determine reactivity, polarity, color, attraction, biological activity, etc. SO3 includes two components mainly - Sulfur and Oxygen. There are one sulfur atom and three oxygen atoms which are spread out as far away as they can!

How to draw SO3 Lewis Structure? Science Education and Tutorials
Sulfur trioxide (alternative spelling sulphur trioxide, also known as nisso sulfan) is the chemical compound with the formula SO 3.It has been described as "unquestionably the most [economically important]" sulfur oxide. It is prepared on an industrial scale as a precursor to sulfuric acid.. Sulfur trioxide exists in several forms - gaseous monomer, crystalline trimer, and solid polymer.
Lewis Dot Structure of the sulfite ion SO32 Electron Dot Structure Chemistry Net
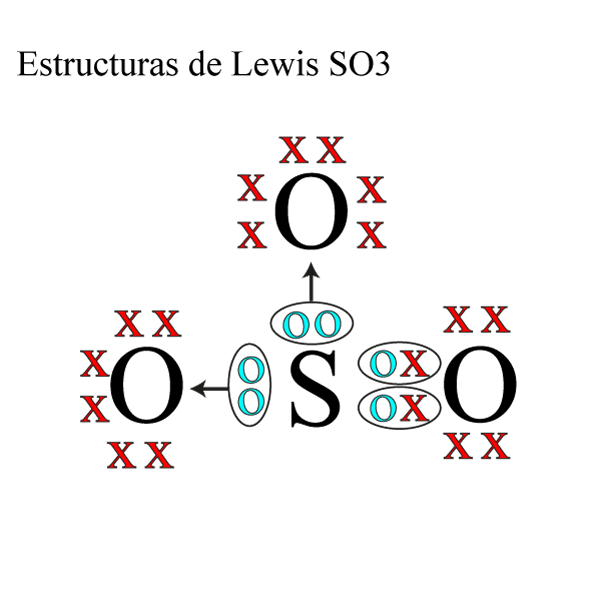
Lewis structure of SO 3 molecule. There are three double bonds around sulfur atom with oxygen atoms in SO molecule. Each oxygen atom has two lone pairs in SO 3 lewis structure. But, there is no lone pair on sulfur atom in SO 3 lewis structure as lewis structure of SO 2.. Hybridization of SO 3 molecule. All atoms have sp 2 hybridization. Each oxygen atom has one sigma bond and two lone pairs.
steps of drawing SO3 lewis structure VSEPR method
Lewis Structure Finder. This widget gets the Lewis structure of chemical compounds. Get the free "Lewis Structure Finder" widget for your website, blog, Wordpress, Blogger, or iGoogle. Find more Chemistry widgets in Wolfram|Alpha.

Estrutura De Lewis So3
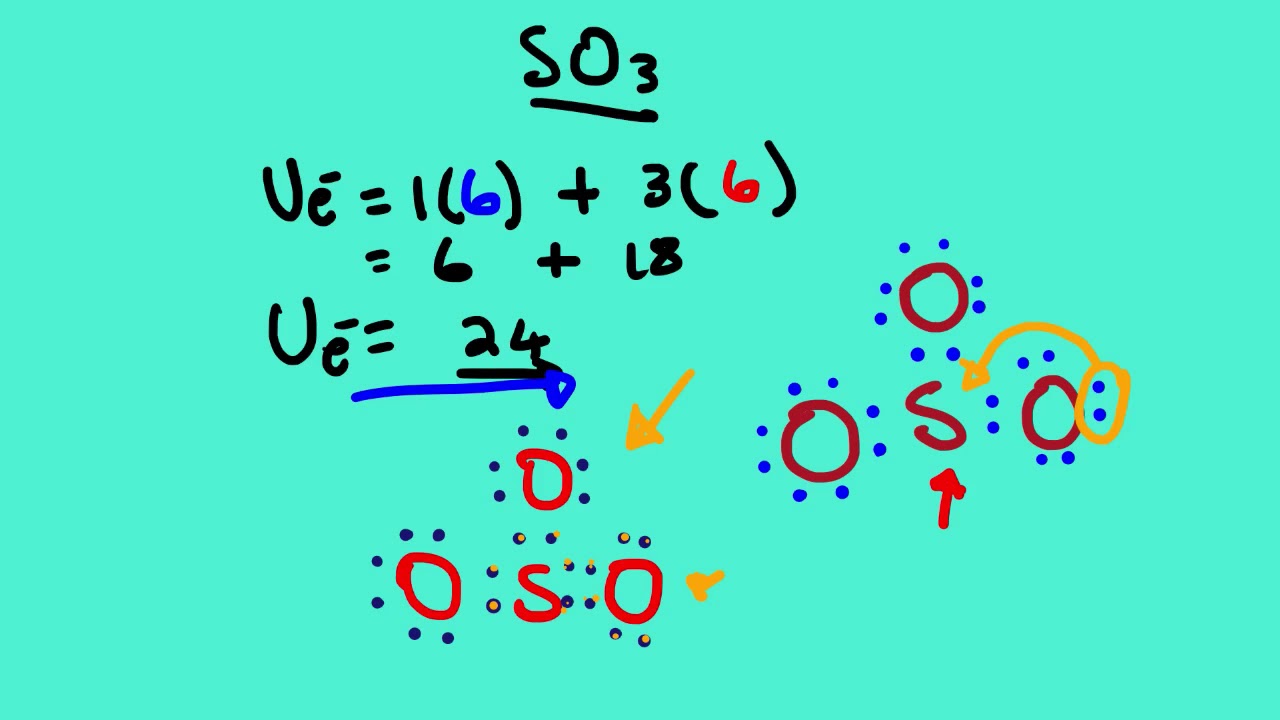
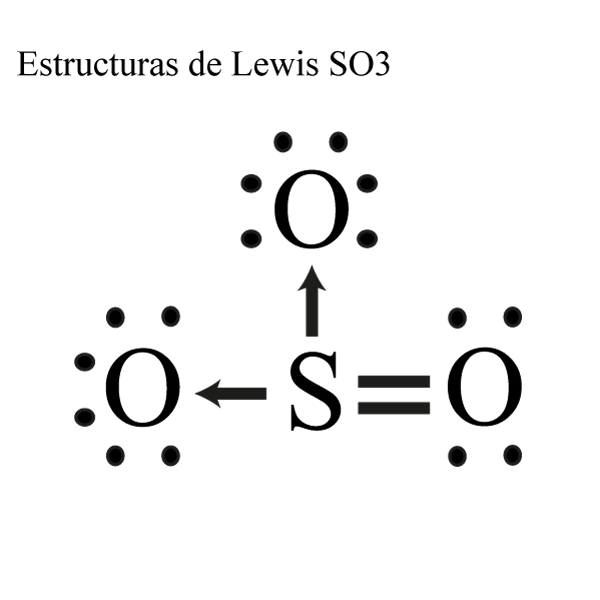
Let's do the SO3 Lewis structure. Sulfur has 6 valence electrons. Oxygen has 6, but we've got three Oxygens, for a total of; 6 plus 18; 24 valence electrons. Let's put Sulfur at the center and then the Oxygens around the outside, all three of them. Now we'll put two valence electrons between each atom to form a chemical bond. We've used 6.
Estructura de Lewis SO3 » Quimica Online
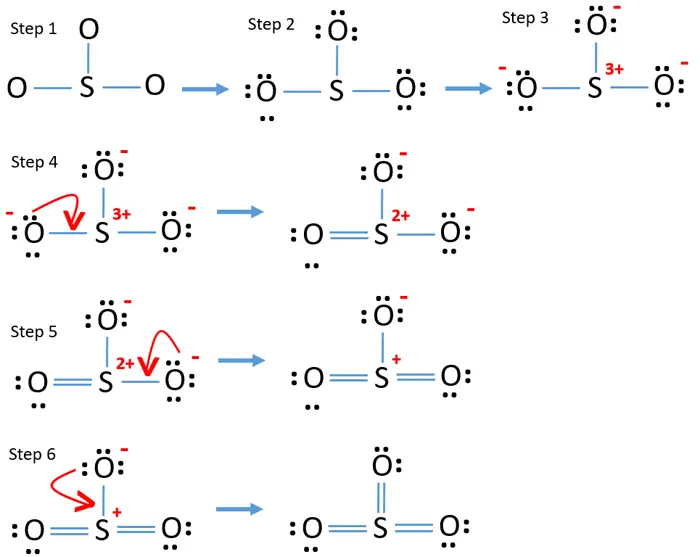
Lewis Structure. The formal charge of an atom in a Lewis structure is a way to determine the distribution of electrons and the stability of the molecule. To calculate the formal charge, we use the formula:. Formal Charge = Valence Electrons - Lone Pairs - 1/2 * Bonded Electrons. In the SO3 Lewis structure, the formal charge of the sulfur atom is 0, while each oxygen atom has a formal.
Estructura de Lewis SO3 » Quimica Online
Website-http://www.kentchemistry.com/links/bonding/LewisDotTutorials/SO3.htmI quickly take you through how to draw the Lewis Structure of SO3 (Sulfur Trioxid.

SO3 Lewis StructureLewis structure of SO3 (Sulfur trioxide) YouTube
A uniform asymptotic analysis of dispersive wave motion. D. S. Ahluwalia E. Reiss S. Stone. Physics. 1974. The signaling problem for the one dimensional Klein-Gordon equation with spatially varying coefficients is analyzed. A formal, uniformly valid, asymptotic expansion of the solution is obtained with…. Expand.

Lewis Dot Structure For So3 slidesharedocs
The chemical formula for sulfur trioxide is SO3. It is a highly reactive compound that is formed by combining sulfur dioxide and oxygen. SO3 is widely used in the production of sulfuric acid, which is an important industrial chemical. The SO3 Lewis structure and its geometry help to understand the bonding, reactivity, and properties of the.
Lewis Dot Diagram For So3 Wiring Diagram
The hybridization of SO3 is sp2. It is determined with the help of formula: Number of hybrid orbitals = Number of sigma bonds + Number of lone pairs. In a single shared double covalent bond, there exists one sigma (σ) bond and one pi (π) bond. So, the total number of sigma bonds in a single SO3 molecule is three, and the total number of lone.

So far, we’ve used 24 of the SO3 Lewis structure’s total 24 outermost valence shell electrons
Step #1: Calculate the total number of valence electrons. Here, the given molecule is SO3 (sulfur trioxide). In order to draw the lewis structure of SO3, first of all you have to find the total number of valence electrons present in the SO3 molecule. (Valence electrons are the number of electrons present in the outermost shell of an atom).

So3 Lewis Structure
The SO3 Lewis structure shows a central Sulfur (S) atom with three Oxygen (O) atoms around it. These atoms are connected by double bonds, and each Oxygen atom has two lone pairs of electrons. In this page, you'll find a detailed, step-by-step guide on how to draw the Lewis structure for SO3. Step-by-Step Guide to Drawing the Lewis Structure.

Calculating SO3 Formal Charges Calculating Formal Charges for SO3 YouTube
How to draw the Lewis Structure of SO3 (sulfur trioxide) - with explanationSulfur is an exception to the octet rule - it can handle up to 12 electrons!Check.

SO3 Molecular Geometry / Shape and Bond Angles (Sulfur Trioxide) YouTube
Drawing the Lewis Structure for SO 3 ( Sulfur Trioxide) SO 3 is the primary contributer to acid rain in the atomsphere. It is a form of pollution. SO 3 is named Sulfur Trioxide. There are 32 valence electrons available for the Lewis structure for SO 3. Be sure to check the formal charges for the Lewis structure for SO 3 .
So3 Lewis Structure Angles
This chemistry video explains how to draw the Lewis structure of SO3 - Sulfur Trioxide. It discusses the molecular geometry, bond angle, hybridization, and.