
Lewis electrondot structure of C2H2 YouTube
Draw a Lewis Structure for C2H2 AND answer the following questions: a. what is the electron group geometry of c2h2? b. what is the molecular geometry of C2H2? c. what is the hybridization of the central atom in C2H2. d. is it polar or nonpolar ? There are 2 steps to solve this one.

C2H2 Geometria Molecular YouTube
Step #1: Calculate the total number of valence electrons. Here, the given molecule is C2H2 (or ethyne or acetylene). In order to draw the lewis structure of C2H2, first of all you have to find the total number of valence electrons present in the C2H2 molecule. (Valence electrons are the number of electrons present in the outermost shell of an.

ChemHelp / Drawing a Lewis dot diagram for acetylene, C2H2 YouTube
C 2 H 2 (acetylene or ethyne) contains two carbon atoms and two hydrogen atoms. There is a triple bond between carbon atoms and hydrogen atoms are joint with carbon atoms though sigma bonds. There are no lone pairs on carbon or hydrogen atoms. In this tutorial, we are going to learn how to draw the lewis structure of C 2 H 2 step by step.

Estrutura De Lewis C2h2
The Lewis structure for C2H2, also known as ethyne or acetylene, is a diagram that shows the arrangement of valence electrons and the bonding between atoms in a molecule. This structure is essential in understanding the properties and behavior of C2H2 in chemical reactions. C2H2 is a hydrocarbon compound made up of two carbon atoms and two.
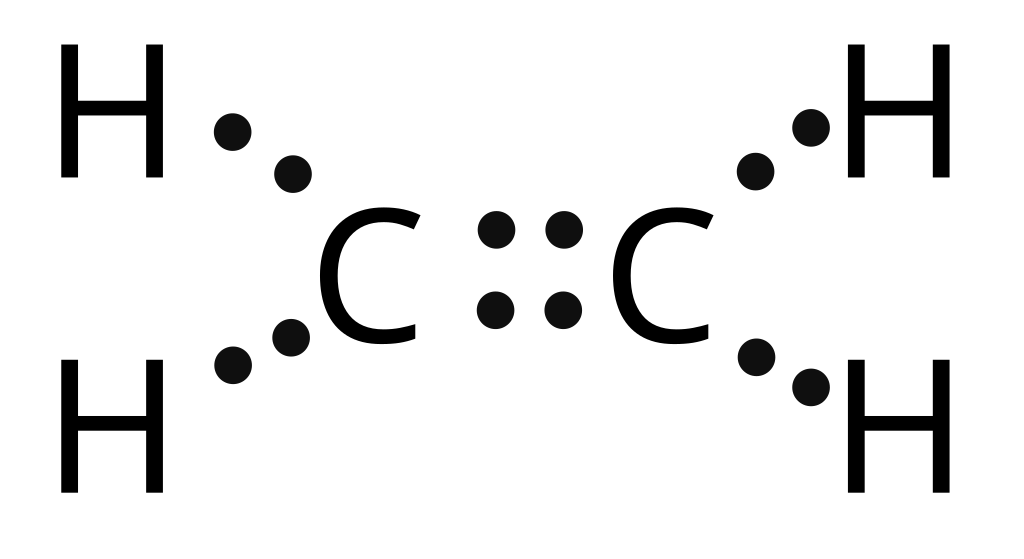
Lewis Dot Diagram For C2h2 Wiring Diagram Pictures
Drawing the Lewis Structure for C 2 H 2 (Ethyne or Acetylene). For C 2 H 2 you have a total of 10 valence electrons to work with.. In drawing the Lewis structure for C 2 H 2 (also called ethyne) you'll find that you don't have enough valence electrons available to satisfy the octet for each element (if you use only single bonds). The solution is to share three pairs of valence electrons and.

C2H2 Lewis Structure, Molecular Geometry, Hybridization, Polarity, and MO Diagram Techiescientist
Struktur Lewis (a) HCN, (b) C2H2, (c) SnO2, (d) BF3, (e) HOF, (f)HCOF, dan (g) NF3

C2H2 Lewis structure ,Valence Electrons, Formal Charge
Question: Below is the Lewis structure of the acetylene (C2H2) molecule H:C::: C:H Count the number of bonding pairs and the number of lone pairs around the left hydrogen atom in this molecule bonding pairs lone pairs: 0 . Show transcribed image text. There are 2 steps to solve this one.

C2h2 Estructura De Lewis Blogan
Learn the steps to draw the Lewis Structure of C2H2 (ethyne or acetylene) in just 1 minute.📌You can draw any lewis structures by following the simple steps.

Estrutura De Lewis C2h2
Lewis Structures. Page ID. A Lewis Structure is a very simplified representation of the valence shell electrons in a molecule. It is used to show how the electrons are arranged around individual atoms in a molecule. Electrons are shown as "dots" or for bonding electrons as a line between the two atoms. The goal is to obtain the "best" electron.
C2H2 Lewis Dot Structure
C2H2 is a linear molecule in form of geometry and the Lewis structure of C2H2 shows that the carbon atom has four valence electrons, while the hydrogen atom has one valence electron as it is an s-block element. Name of Molecule. Acetylene or ethyne. Chemical Formula. C2H2.

Estructura De Lewis Del C2h2 Compuesto
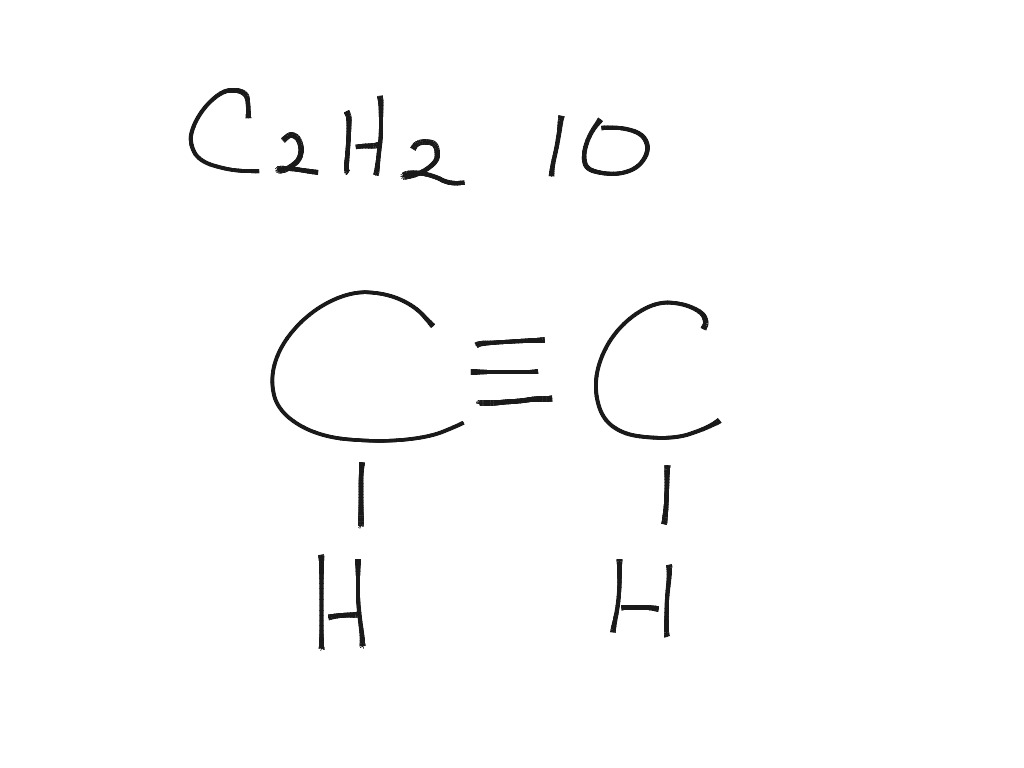
The total number of valence electrons in the acetylene or ethyne (C2H2) Lewis dot structure is 10. The molecular geometry or shape of C 2 H 2 is identical to its ideal electron pair geometry i.e., linear. The bonded atoms in C 2 H 2 form a mutual bond angle of 180°. The central C-atoms have sp hybridization in C 2 H 2.

Estrutura De Lewis C2h2
Use these steps to correctly draw the C 2 H 2 Lewis structure: #1 First draw a rough sketch. #2 Mark lone pairs on the atoms. #3 Calculate and mark formal charges on the atoms, if required. #4 Convert lone pairs of the atoms, and minimize formal charges.

Lewis Dot Diagram For C2h2
I quickly take you through how to draw the Lewis Structure of CHCH (Acetylene or ethyne). I also go over hybridization, shape, sigma, pi bonding and bond ang.

C2H2 Lewis Structure, Molecular Geometry, Hybridization, Polarity, and MO Diagram Techiescientist
C2H2 Lewis Structure. Lewis Structure of any molecule helps to know the arrangement of all atoms, their valence electrons, and the bond formation in the molecule. The electrons that participate in forming bonds are called bonding pairs of electrons while the ones that do not take part in any bond formation are called lone pairs or non-bonding pairs of electrons.
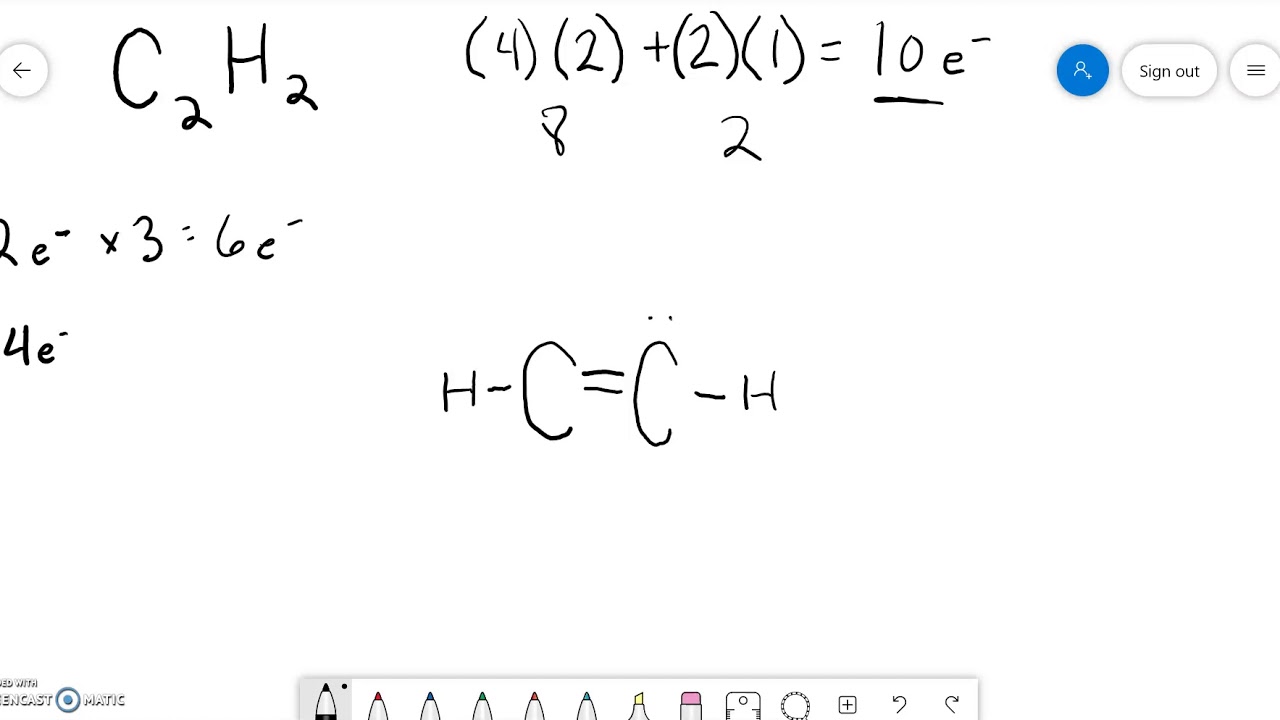
Lewis Structure C2H2 YouTube
Acetylene (systematic name: ethyne) is the chemical compound with the formula C 2 H 2 and structure H−C≡C−H.It is a hydrocarbon and the simplest alkyne. This colorless gas is widely used as a fuel and a chemical building block. It is unstable in its pure form and thus is usually handled as a solution. Pure acetylene is odorless, but commercial grades usually have a marked odor due to.

C2H2 Molecular Geometry,Shape and Bond Angles(Acetylene) Molecular geometry, Geometry, Molecular
Step 1: Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. Step 2: Connect the atoms to each other with single bonds to form a "skeleton structure.".