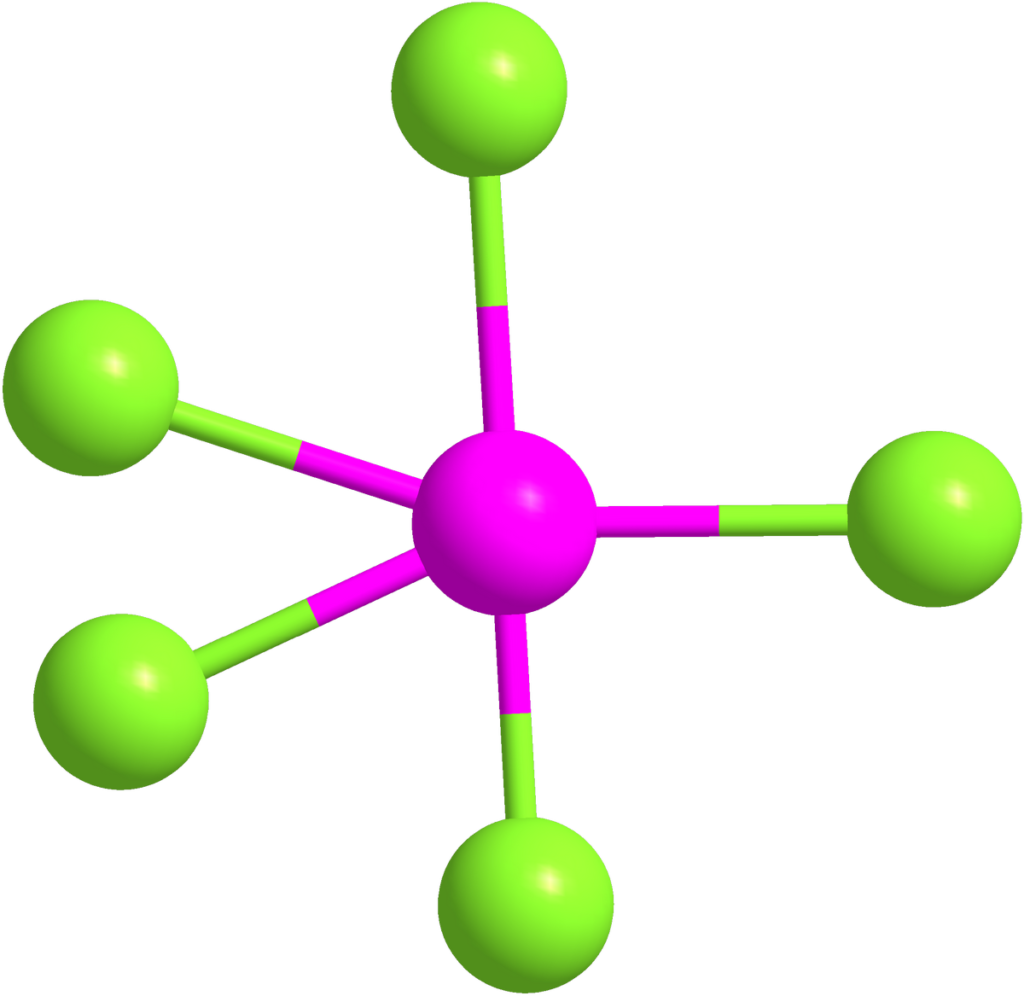
Bentuk Molekul PCl5 MateriKimia
PCl5 Lewis Structure Step-by-Step Guide. 1. Determine the total number of valence electrons in PCl5. Phosphorus (P) is in Group 5 of the periodic table, so it has 5 valence electrons. Chlorine (Cl) is in Group 7, so it has 7 valence electrons. Since there are five chlorine atoms, the total number of valence electrons is 5 (from P) + 7 (from.

PCl5 Lewis Structure, Molecular Geometry, Hybridization, and MO Diagram Techiescientist
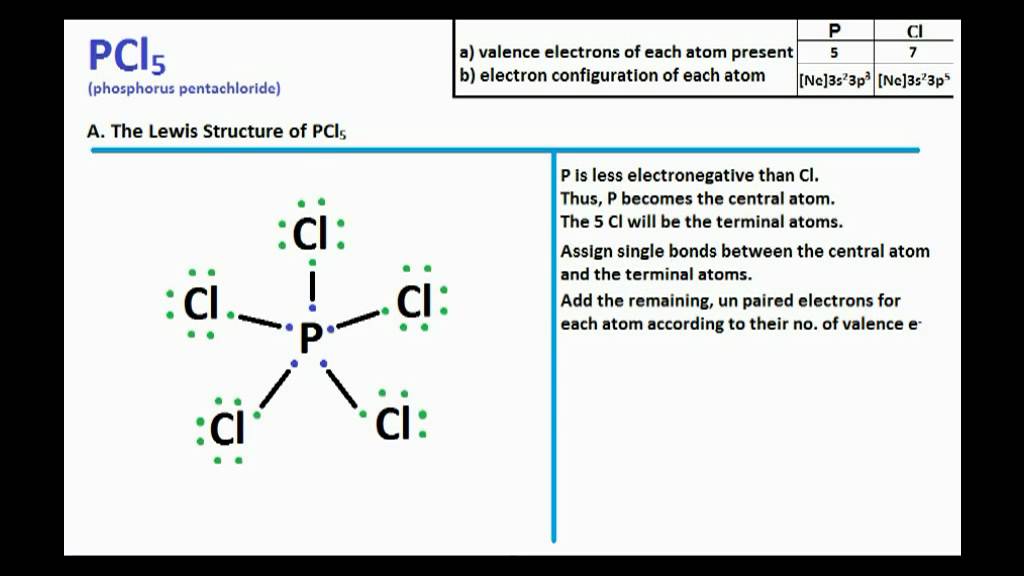
Another (easier) method to determine the Lewis structure of PCl 5: Alternatively a dot method can be used to draw the Lewis structure. Calculate the total valence electrons in the molecule. P: 1×5 = 5. Cl: 5×7 = 35. Total = 40 valence electrons. Now, treat the atoms and electrons like puzzle pieces.

PCl5 lewis structure, molecular geometry, bond angle, hybridization
Drawing the Lewis Structure for PCl 5. PCl 5 is similar to PBr 5 and PF 5. If you can do those Lewis structures PCl 5 will be easy. In the PCl 5 Lewis structure Phosphorus (P) is the least electronegative so it goes in the center. In the Lewis structure for PCl 5 there are a total of 40 valence electrons. Five pairs will be used in the chemical.
PCl5 Lewis Structure and Molecular Geometry YouTube
in this video you will learn how to draw Lewis structure (Lewis formula) for PCl5 (Phosphorous Pentachloride).Lewis structure Playlist: https://www.youtube.c.
William Of Wales pcl5 lewis structure
I quickly take you through how to draw the Lewis Structure of PCl5, phosphorous pentachloride. I also go over formal charge, hybridization, shape and bond an.

Lewis Structure Pcl5
MO diagram depicts chemical and physical traits of a molecule like bond length, bond energy, bond angle, shape, etc. Following are the steps to design the MO diagram of PCl5 : Step 1: Identify the valence electrons of each atom. In PCl5, it is 5 for P and 7 for every 5 atoms of Cl. Step 2: Check if the molecule is heteronuclear or homonuclear.
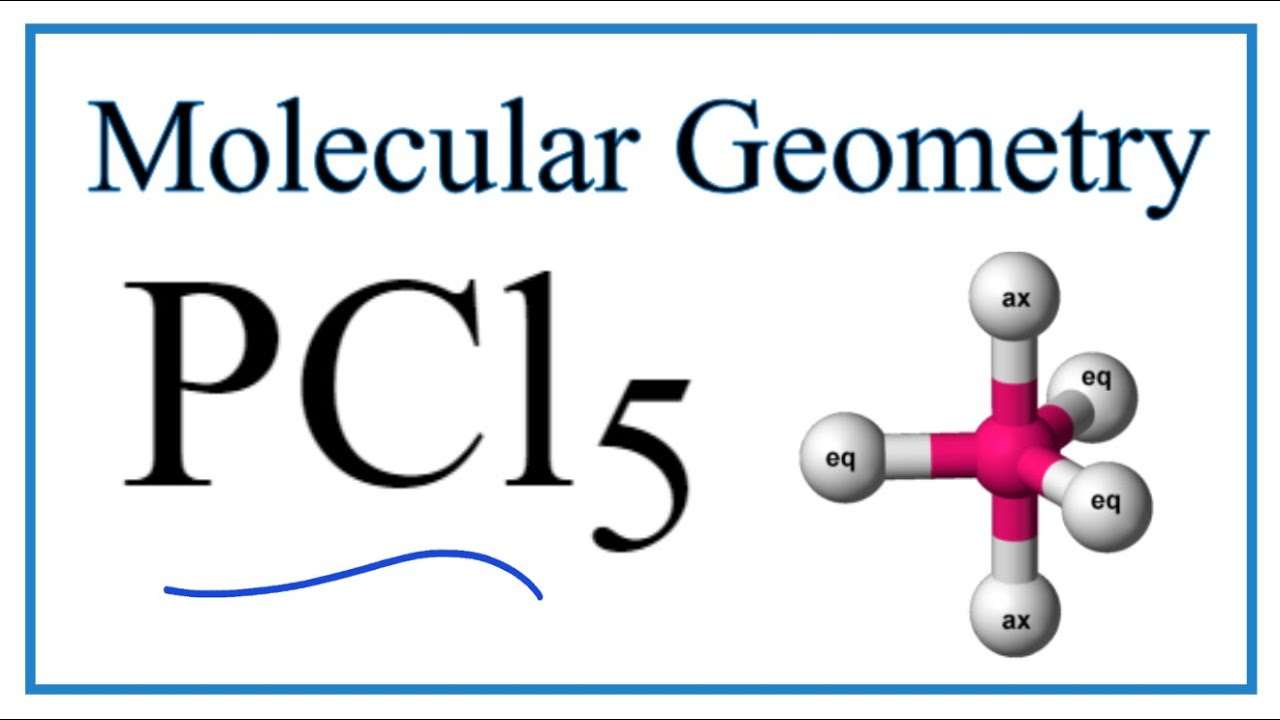
PCl5 (Phosphorus pentachloride) Molecular Geometry, Bond Angles YouTube
Steps of drawing PCl5 lewis structure Step 1: Find the total valence electrons in PCl5 molecule. In order to find the total valence electrons in PCl5 (phosphorus pentachloride) molecule, first of all you should know the valence electrons present in phosphorus atom as well as chlorine atom. (Valence electrons are the electrons that are present in the outermost orbit of any atom.)
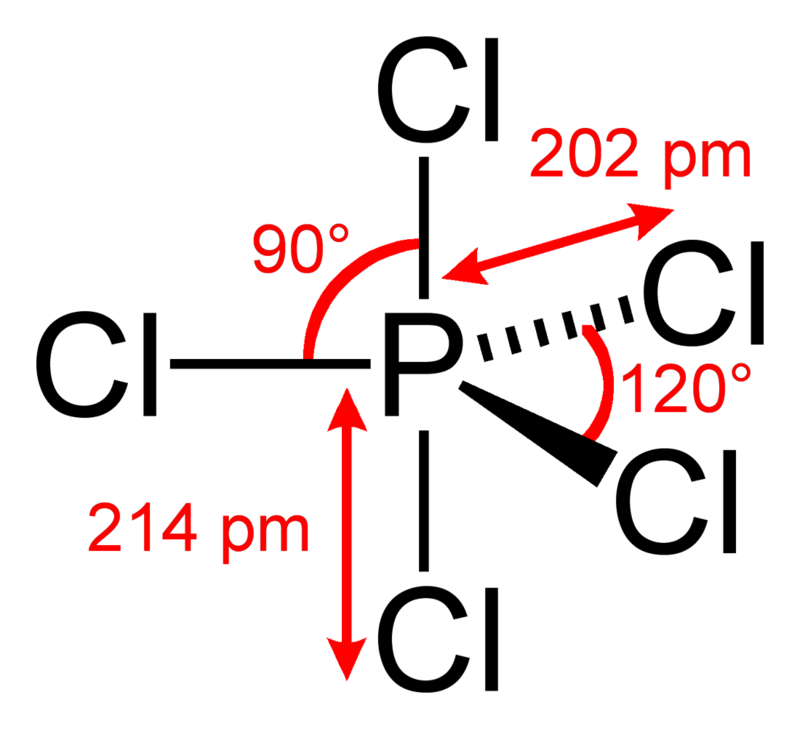
What is the structure of PCl_5? + Example
Check me out: http://www.chemistnate.com
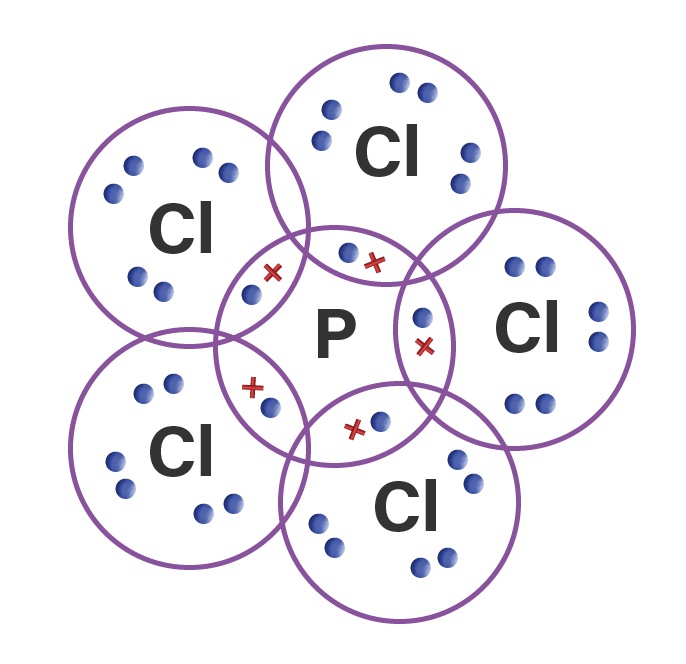
Gambarkan rumus Lewis dari molekul PCl5
Step #1: Calculate the total number of valence electrons. Here, the given molecule is PCl5 (Phosphorus pentachloride). In order to draw the lewis structure of PCl5, first of all you have to find the total number of valence electrons present in the PCl5 molecule. (Valence electrons are the number of electrons present in the outermost shell of an.

QUIMICA Estructura de Lewis PCl5 Carga Formal e hibridación sp3d Expansión Octeto AULAEXPRESS
Steps for drawing PCl5 lewis structure. Let's draw the lewis dots for PCl 5 by following the below steps one by one: Find how many number of valence electrons are available for bonding in PCL 5. P (Z = 15) = [Ne] 3s²3p³ ie. 5 valence electrons are there for Phosphorous. Cl (Z = 17) = [Ne] 3s²3p⁵ ie. 7 electrons for each chlorine atom.

25. Lewis Dot Structure of PCl5 How to Draw Lewis Structures Class 11 Chemistry Chemical
Steps. Use these steps to correctly draw the PCl 5 Lewis structure: #1 First draw a rough sketch. #2 Mark lone pairs on the atoms. #3 Calculate and mark formal charges on the atoms, if required. Let's discuss each step in more detail.

Pcl5 Lewis Structure / Figure 5.5 lewis symbols for the elements of the first three periods
Step 1: Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. Step 2: Connect the atoms to each other with single bonds to form a "skeleton structure.".

PCl5 lewis structure, molecular geometry, hybridization, bond angle
Phosphorus pentachloride is the chemical compound with the formula PCl 5. It is one of the most important phosphorus chlorides/oxychlorides, others being PCl 3 and POCl 3. PCl 5 finds use as a chlorinating reagent. It is a colourless, water-sensitive solid, although commercial samples can be yellowish and contaminated with hydrogen chloride .

Pembahasan soal Struktur Lewis Bentuk molekul PCl5 YouTube
PCl. 5. (Phosphorus pentachloride) Lewis Structure. Phosphorus pentachloride (PCl 5) contains five chlorine atoms and one phosphorus atom. In PCl 5 lewis structure, each chlorine atom is joint with center phosphorus atom through a single bond (sigma bond). You can see there is no lone pairs on phosphorus atom in PCl 5 as PCl 3.

Kovalen Rangkap serta Penyimpangan Kaidah Oktet Kimia Kelas 10
In the PCl 5 Lewis dot structure, a total of 15 lone pairs and 5 bond pairs are present. The electron geometry of PCl 5 is also Trigonal bipyramidal. The hybridization of phosphorous in PCl 5 is sp 3 d. Since its steric number is 5. In PCl 5, axial atoms (2 P-Cl bonds) make a 90º angle with the plane, and equatorial atoms (3 P-Cl bonds) make a.

Pcl5 Lewis Structure
Lewis Structures. Page ID. A Lewis Structure is a very simplified representation of the valence shell electrons in a molecule. It is used to show how the electrons are arranged around individual atoms in a molecule. Electrons are shown as "dots" or for bonding electrons as a line between the two atoms. The goal is to obtain the "best" electron.