
So2 electron geometry mintdop
Find 89 houses for sale in Pont-Rouge, QC. Visit REALTOR.ca to see all the Pont-Rouge, QC real estate listings on the MLS® Systems today! Prices starting at $40,000 💰

Estructura de Lewis SO2 » Quimica Online
This is a theoretical structure obtained using formal charges- this is the structure that we will take to be Sulfur Dioxide's final Lewis structure. However, it is worth noting that in an experimental sense (data and tools), we find single and double bonds present in the SO 2 structure. The final Lewis structure for SO 2 is shown below. It.

SO2 Molecular Geometry Science Education and Tutorials
2. ) Lewis Structure, Hybridization. Sulfur dioxide molecule contains one sulfur atom and two oxygen atoms. We will construct the lewis structure of SO 2 molecule by following VSEPR theory rules and considering stability of intermediate structures. After obtaining the lewis structure of SO 2, we can determine the hybridization of atoms.
SO2(Sulfur Dioxide) Molecular Geometry & Lewis Structure Geometry of Molecules
Get the free "Lewis Structure Finder" widget for your website, blog, Wordpress, Blogger, or iGoogle. Find more Chemistry widgets in Wolfram|Alpha.
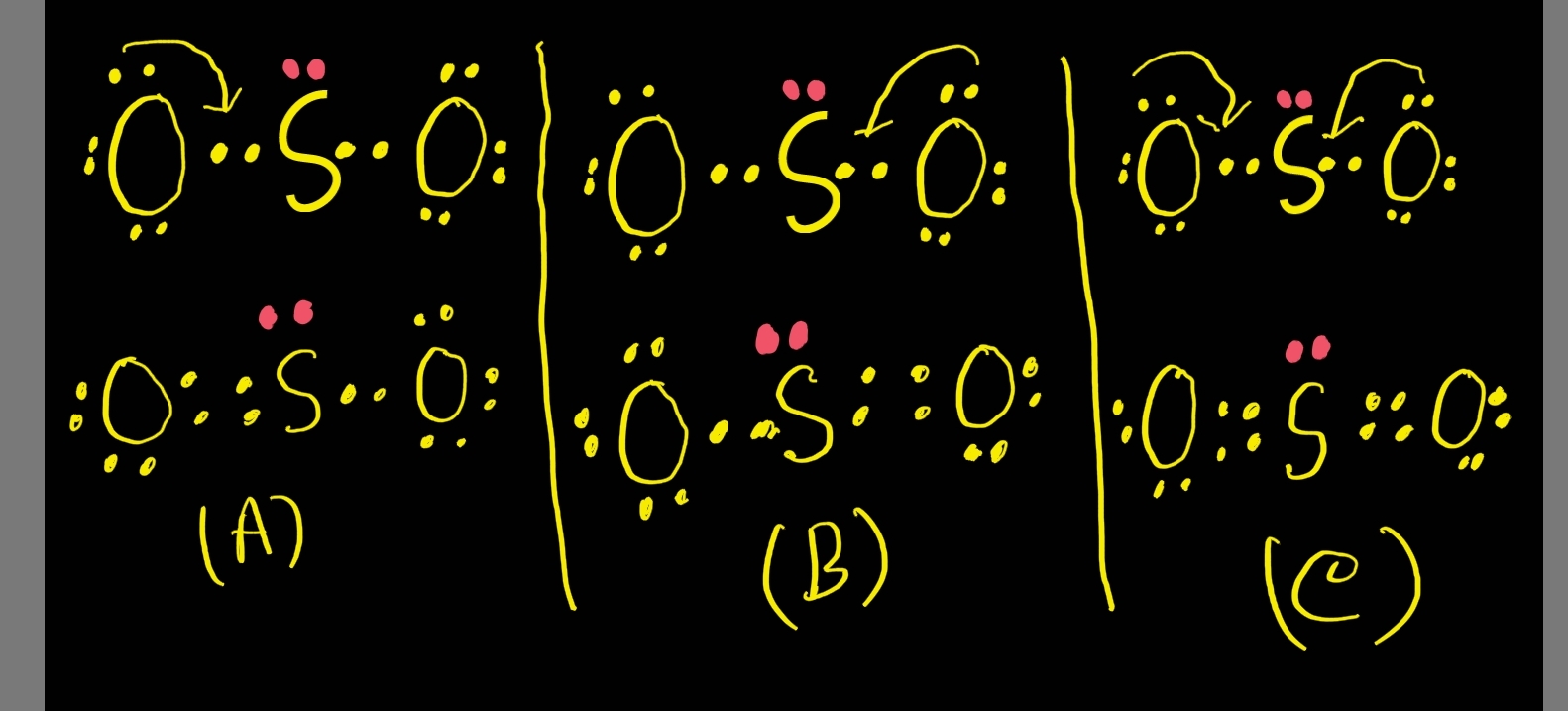
SO2 Lewis Structure ,Valence Electrons ,Formal Charge,Polar or Nonpolar
Sulfur dioxide is spelled as Sulphur dioxide in Commonwealth English. This is a pungent-smelling, colorless gas. Talking about its properties, SO2 has a molar mass of 64.066 g/mol. The melting point and boiling points are -72℃, and -10℃ respectively. Now let's move on to the fundamental concepts like lewis structure, molecular geometry.

SO2 Lewis Structure ,Valence Electrons ,Formal Charge Lewis Structure for SO2 (Sulfur Dioxide)
A step-by-step explanation of how to draw the SO2 Lewis Structure (Sulfur Dioxide) Note: From an experimental view (using x-ray crystallography or someth.
SO2(Sulfur Dioxide) Molecular Geometry & Lewis Structure Geometry of Molecules
Step 1: Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. Step 2: Connect the atoms to each other with single bonds to form a "skeleton structure.".

SO2 Lewis Structure ,Valence Electrons ,Formal Charge,Polar or Nonpolar
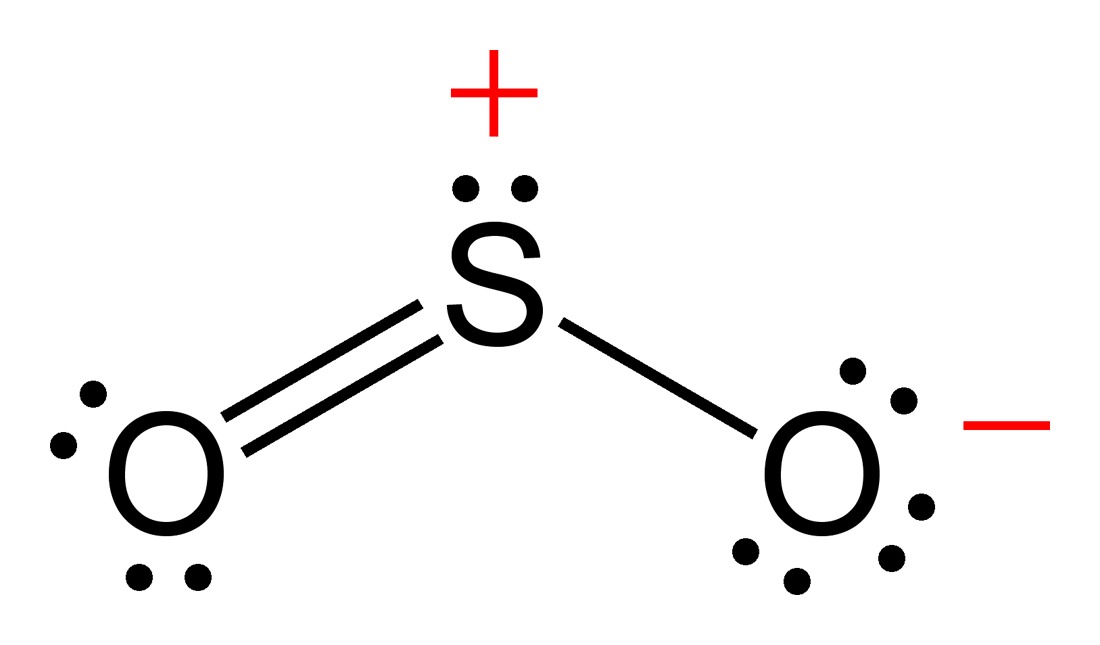
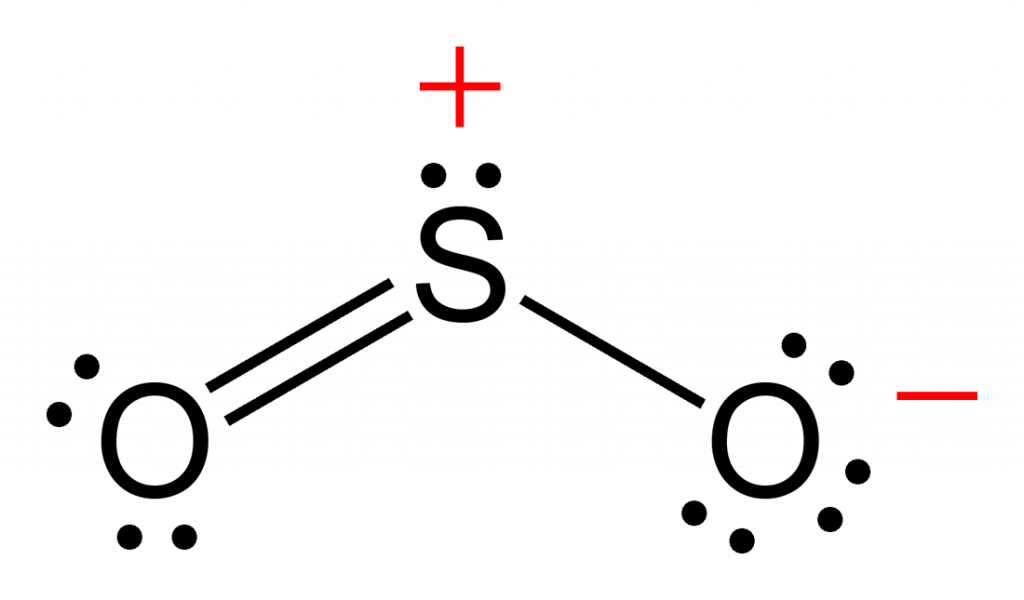
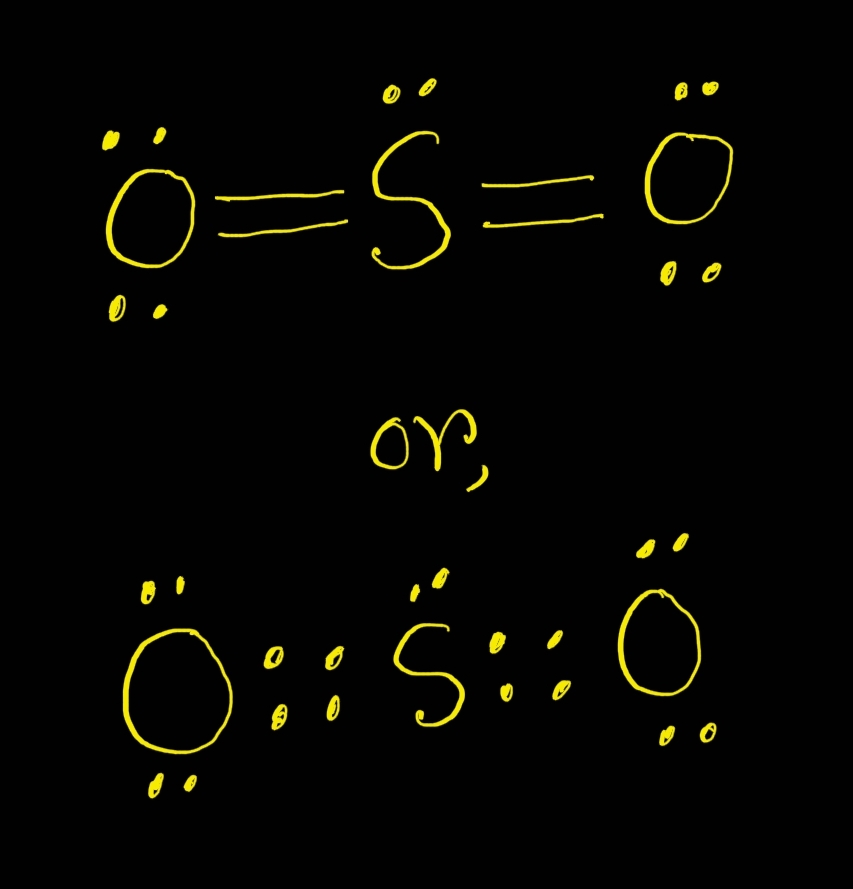
A sulfur atom (S) and two oxygen atoms (O) make up the SO2 Lewis structure. The sulfur atom (S) is the center atom, and the two oxygen atoms (O) surround it at a bond angle of 119 degrees. The sulfur atom (S) and each oxygen atom (O) form two double bonds. The two oxygen atoms (O) each have two lone pairs, while the sulfur atom (S) has one..
SO2 Lewis Structure ,Valence Electrons ,Formal Charge Lewis Structure for SO2 (Sulfur Dioxide)
Step #1: Calculate the total number of valence electrons. Here, the given molecule is SO2 (sulfur dioxide). In order to draw the lewis structure of SO2, first of all you have to find the total number of valence electrons present in the SO2 molecule. (Valence electrons are the number of electrons present in the outermost shell of an atom).
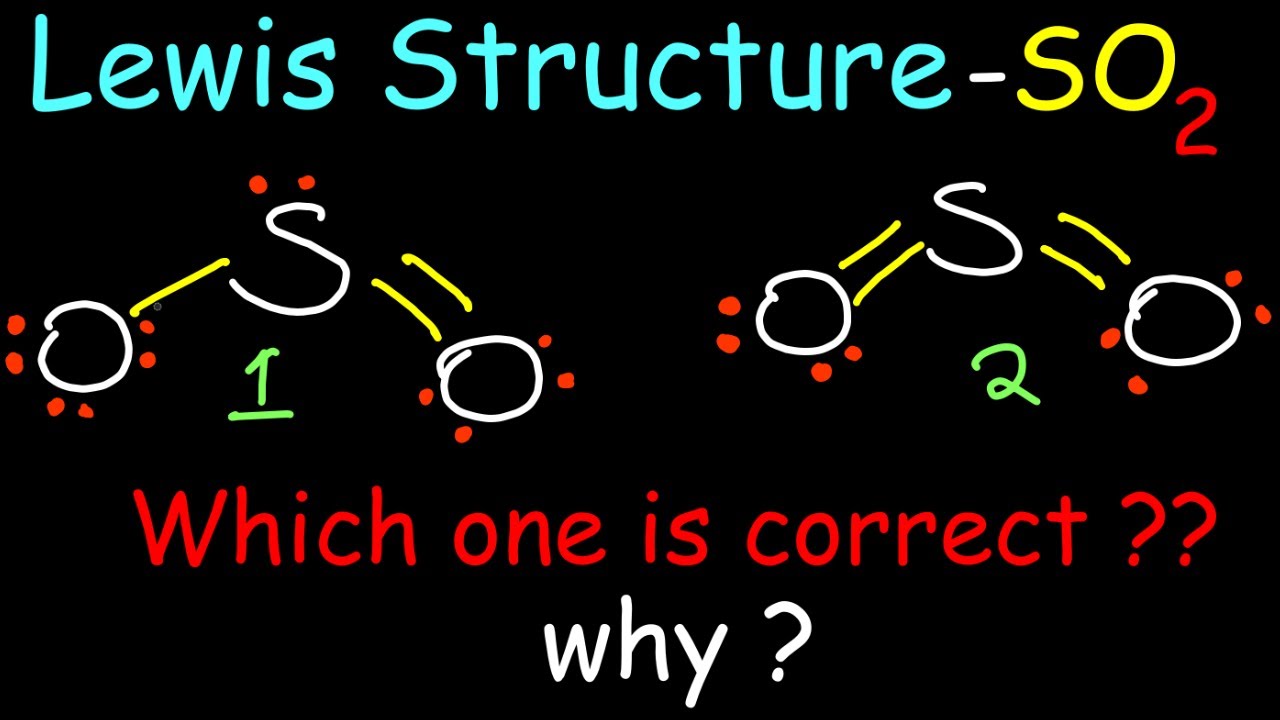
Lewis Structure of Sulphur Dioxide SO2 YouTube
Drawing the Lewis Structure for SO 2. The Lewis structure for SO 2 requires you to place more than 8 valence electrons on Sulfur (S). You might think you've got the correct Lewis structure for SO 2 at first. Remember, Sulfur is in Period 3 and can hold more than 8 valence electrons. You'll want to calculate the formal charges on each atom to.
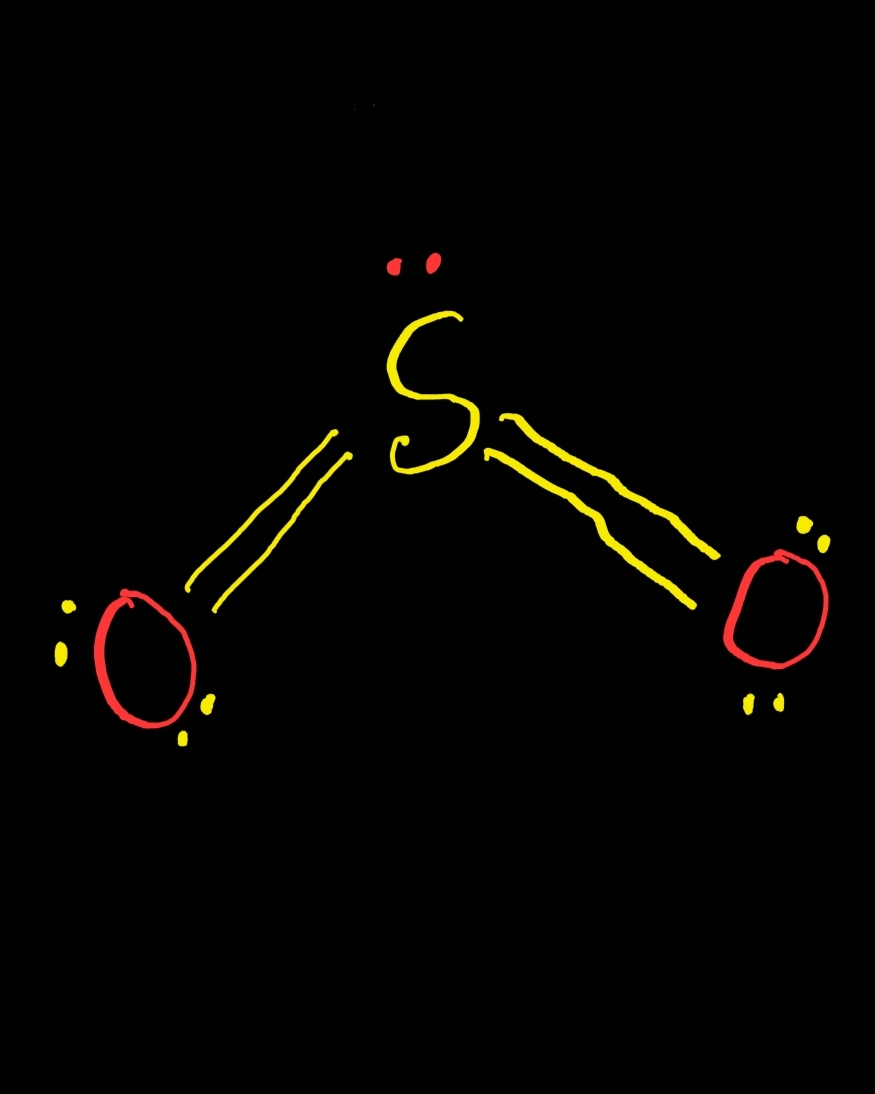
SO2 Lewis Structure ,Valence Electrons ,Formal Charge Lewis Structure for SO2 (Sulfur Dioxide)
The SO 2 Lewis structure depicts the molecular arrangement of sulfur dioxide, which consists of one sulfur atom and two oxygen atoms. In the SO 2 Lewis structure, there is a double bond between the sulfur atom and each oxygen atom. Furthermore, each oxygen atom possesses two lone pairs, and the sulfur atom has one lone pair. To draw the SO 2 Lewis structure correctly, begin by sketching the.

SO2 Molecular Geometry,Shape and Bond Angles (Sulfur Dioxide) Bioquímica, Química, Atomo
In this video, we'll learn about the Lewis Structure of Sulphur Dioxide - SO2. This structure is key to understanding the chemistry of Sulphur Dioxide. We'll.
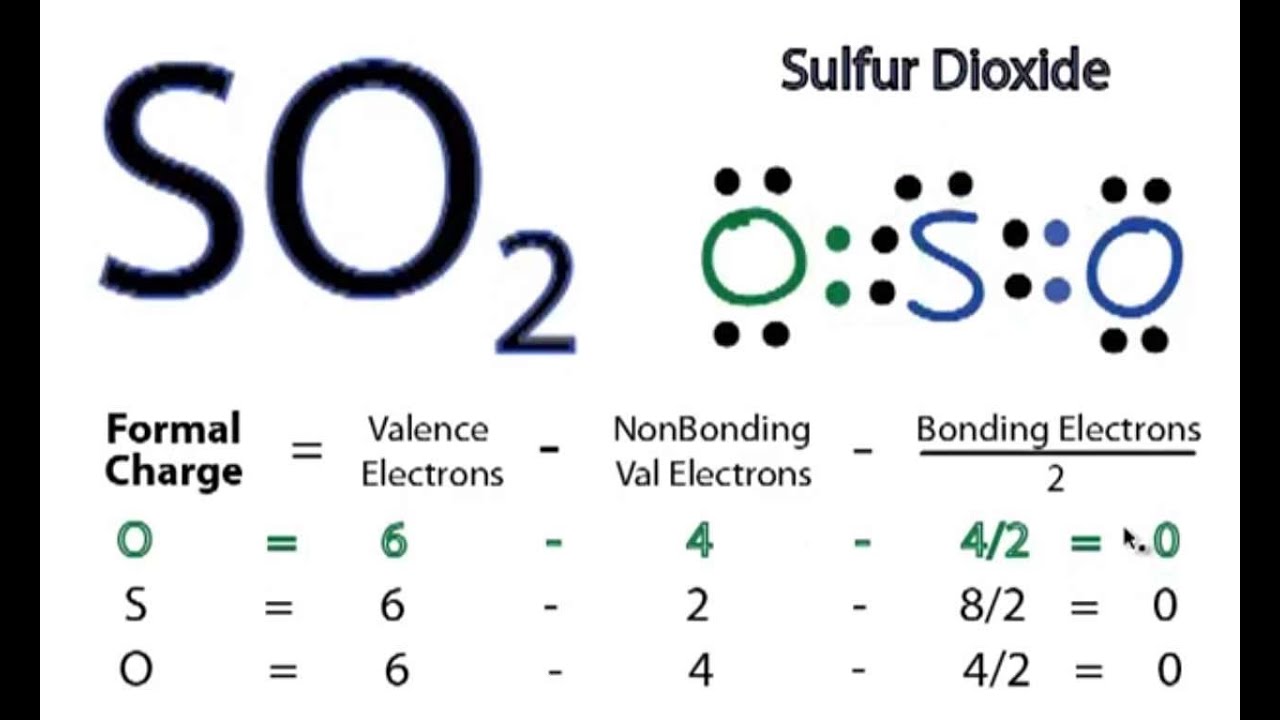
SO2 Lewis Structure How to Draw the Lewis Structure for SO2 (Sulfur Dioxide) YouTube
Lewis Structure of SO2 (sulfur dioxide) - YouTube
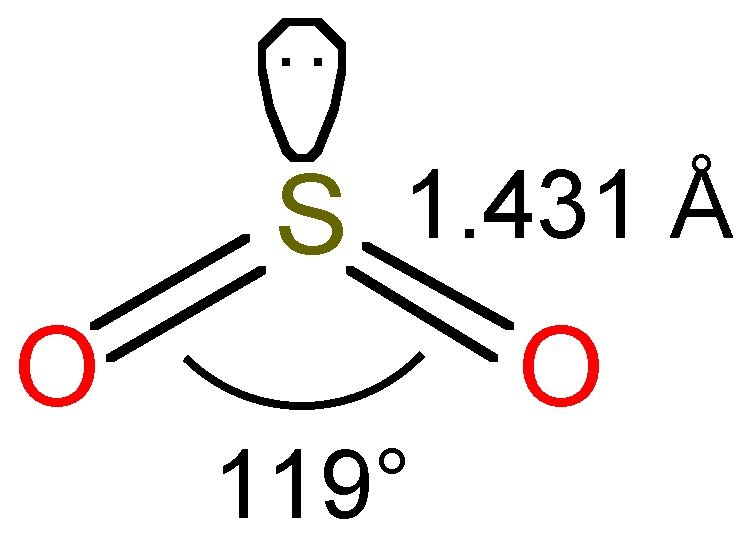
SO2(Sulfur Dioxide) Lewis Structure, Hybridization, Molecular Geometry, and Bond Angles
SO2 Lewis Structure Step-by-Step Guide. 1. Determine the total valence electrons. Start by counting the valence electrons of each atom in the molecule. In SO2, sulfur is in Group 6, so it has 6 valence electrons, while each oxygen atom in Group 6 contributes 6 valence electrons. Therefore, the total valence electrons in SO2 are 6 + 2 (6) = 18. 2.
SO2 Lewis Structure ,Valence Electrons ,Formal Charge,Polar or Nonpolar
SO2 lewis structure of total valence electrons 18.Sulfur and oxygen has six electrons. sulfur has six valence electrons, 2 non bonding and 6 bonding electrons. Six bonding electrons divided by 2 , we get 3 electrons. So the Formal charge of sulfur is 6-2-3 =+1. One of the oxygen having formal charge +1.

How to draw SO2 Lewis Structure? Science Education and Tutorials
Conclusion. SiO2 has a net dipole moment of zero. It has a linear electron and molecular geometry with a bond angle of 180 degrees and a hybridization of Sp. The Silicon dioxide Lewis structure has a total of 16 valence electrons. In the Lewis dot structure of SiO2, the formal charge is zero.