
PPT Teori atom Thomson PowerPoint Presentation, free download ID5672739
Teori atom J.J. Thomson adalah salah satu titik awal dalam perkembangan pemahaman manusia tentang struktur atom. Meskipun model "kue krim (plum pudding)" atau model atom seperti roti kismis, atom Thomson memberikan pemahaman awal yang penting, model ini juga memiliki kelebihan dan kelemahan yang perlu dipertimbangkan.
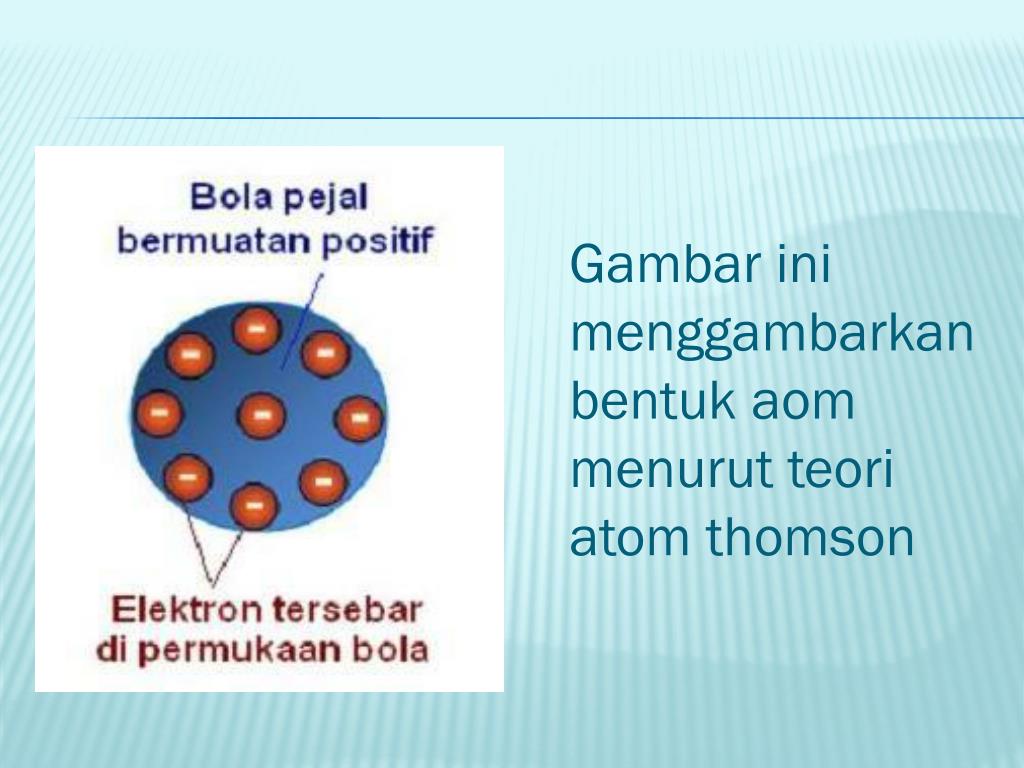
PPT Teori atom Thomson PowerPoint Presentation, free download ID5672739
Penelitiannya membuahkan penemuan elektron. Thomson mengetahui bahwa gas mampu menghantar listrik. Ia menjadi perintis ilmu fisika nuklir. Thomson memenangkan Hadiah Nobel Fisika pada tahun 1906 . Biografi Joseph John Thomson lahir di Creetham Hill, pinggiran kota Manchester pada tanggal 18 Desember 1856.
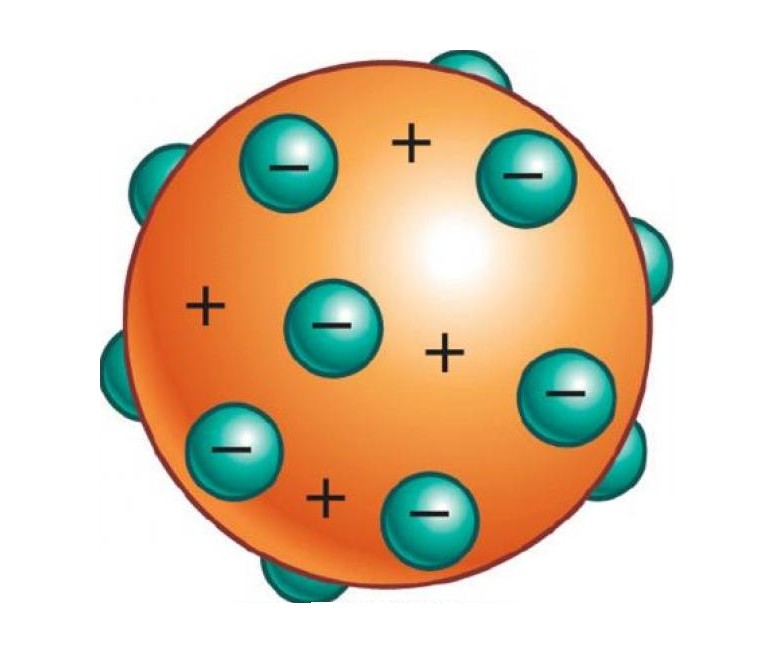
Bunyi Teori Model Atom Joseph John Thomson serta Gambar Ruana Sagita
Thomson atomic model, earliest theoretical description of the inner structure of atoms, proposed about 1900 by William Thomson (Lord Kelvin) and strongly supported by Sir Joseph John Thomson, who had discovered (1897) the electron, a negatively charged part of every atom.

PPT Teori atom Thomson PowerPoint Presentation, free download ID5672739
Early Life and Education. Joseph John Thomson, who was always called J.J., was born in Cheetham Hill, England, near Manchester, in 1856. His father was a bookseller who planned for Thomson to be.

Thomson atomic model Description, Plum Pudding, & Image Britannica
ATOMISM. A term deriving from the Greek ἄ τ ο μ ο ν, meaning indivisible, and usually applied to systems maintaining that everything is composed of unchanging and indivisible elements or atoms, whose movements and arrangements account for the changing appearances of reality.In a broader sense, the term is applied also to any systematic explanation that attempts to reduce complex.

Thomson’s Atomic Model Structure of Atom Class 9 Chemistry YouTube
atom or electron atomic number atomic quality Atomic Theory atomic weight Barkla has shown cathode rays change in mass chemical affinity chemical combination chemical compounds chemical elements chemists chlorine compound connexion Democritus depend detected determine the number different elements drops of water dynamical quantities effective.

Fisika Atom Kelas 12 • Part 1 Teori Atom Thomson dan Rutherford YouTube
The British scientist Sir Joseph Thomson is best known in the world of physics for discovering the electron. Thomson studied and taught mathematics, physics, and chemistry at Trinity College, Cambridge University, from 1876 until his death in 1940. He became director of the Cavendish Laboratory at the early age of 27.

Perkembangan Teori Atom dan TokohTokohnya Gramedia Literasi
In 1903, Thomson proposed a model of the atom consisting of positive and negative charges, present in equal amounts so that an atom would be electrically neutral. He proposed the atom was a sphere, but the positive and negative charges were embedded within it.

PPT Teori atom Thomson PowerPoint Presentation, free download ID5672739
Written by Kamal N Perkembangan model atom - Partikel paling kecil yang menjadi penyusun materi pertama kali ditemukan oleh dua orang filsafat Yunani yaitu Leucippus dan Democritus pada sekitar 450 tahun Sebelum Masehi. Kedua filsuf Yunani tersebut mengungkapkan, bahwa seluruh materi tersusun oleh partikel terkecil dan tidak dapat dibagi.
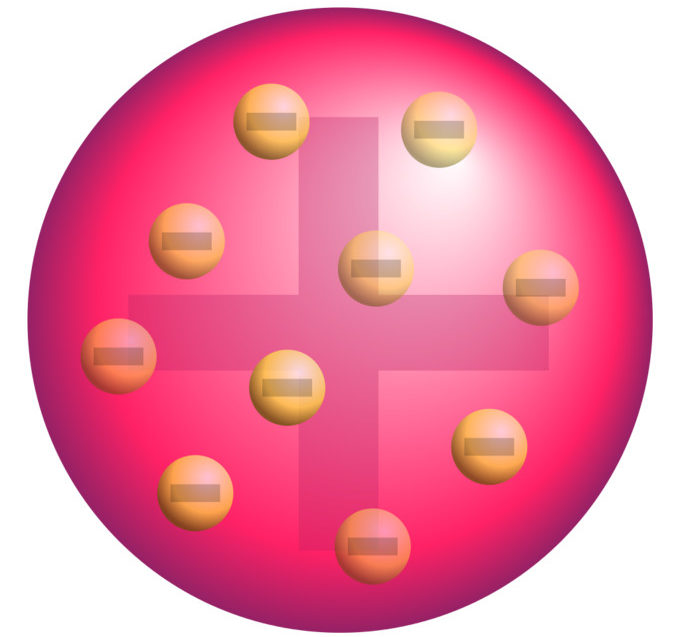
Model Atom Thomson
About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy & Safety How YouTube works Test new features NFL Sunday Ticket Press Copyright.
What is Thomson's atom? Socratic
Teori model atom Thomson pertama kali diusulkan pada tahun 1898 oleh fisikawan Inggris Joseph John Thomson atau hanya JJ Thomson. Setelah memiliki beberapa bukti eksperimental tentang keberadaan elektron, ia merombak teori atom yang tidak dapat dibagi yang dikemukakan oleh John Dalton.

Teori Atom Menurut Joseph John Thomson Ruana Sagita
The British physicist Joseph John "J. J." Thomson (1856-1940) performed a series of experiments in 1897 designed to study the nature of electric discharge in a high-vacuum cathode-ray tube, an area being investigated by many scientists at the time. Thomson interpreted the deflection of the rays by electrically charged plates and magnets.
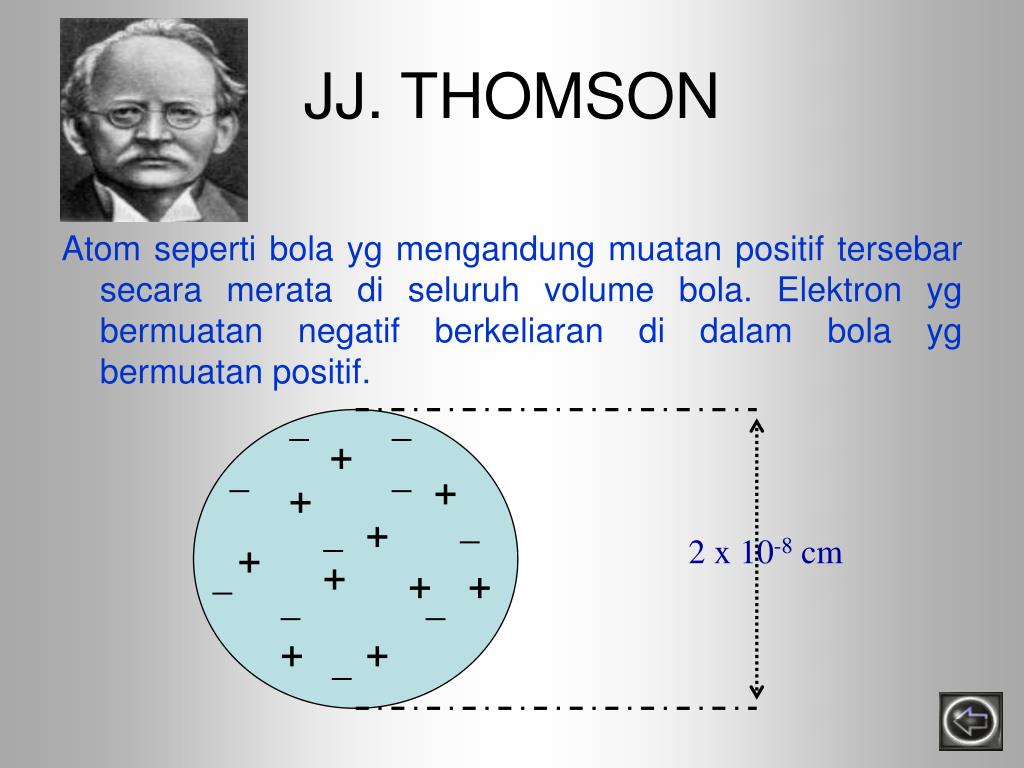
PPT Perkembangan Teori Atom PowerPoint Presentation, free download ID3938297
J.J. Thomson (born December 18, 1856, Cheetham Hill, near Manchester, England—died August 30, 1940, Cambridge, Cambridgeshire) English physicist who helped revolutionize the knowledge of atomic structure by his discovery of the electron (1897). He received the Nobel Prize for Physics in 1906 and was knighted in 1908.. Education and early career. Thomson was the son of a bookseller in a.

PPT Teori atom Thomson PowerPoint Presentation, free download ID5672739
Teori atom yang dikemukakan oleh J.J Thomson menitikberatkan pada atom sebagai bola masif bermuatan positif yang di dalamnya tersebar elektron sehingga keseluruhannya bersifat netral. Baca juga: Kelebihan dan Kekurangan Teori Atom Rutherford

J. J. Thomson
Pada masa awal perkembangan teori atom, Dalton beranggapan bahwa atom adalah zat terkecil yang tidak dapat dibagi lagi. Baca juga: Sejarah Penemuan Proton, Elektron, Neutron, dan Inti Atom Dilansir dari Lumen Learning, hal tersebut patah ketika Thomson menemukan elektron pada tahun 1897 dan mengusulkan model atom Thomson.

Perkembangan Teori Atom Democritus, Aristoteles, Dalton, Thomson, Rutherford, Niels Bohr
1. Teori Atom Dalton (John Dalton) 2. Teori Atom Thomson (Sir Joseph John Thomson) 3. Teori Atom Rutherford (Ernest Rutherford) 4. Teori Atom Bohr (Niels Bohr) 5. Teori Atom Mekanika Kuantum (Werner Heisenberg dan Erwin Schrödinger) Kesimpulan Rekomendasi Buku & Artikel Terkait Kategori Ilmu Kimia Materi Terkait Struktur Atom